Pablo Olea-Rodríguez¹, Juan Jesús Pérez-Núñez¹.
- Faculty of Medicine, University of Granada (UGR)
TRANSLATED BY:
Irene Torres-Martínez², Paola Rodríguez-González², Mercedes Ortigosa-García², Alberto González-Ramírez², Carmen
Salmerón-Borja². - Faculty of Translation and Interpreting, University of Granada (UGR).
Abstract
Introduction
Prostate cancer is one of the most prevalent cancers among men. An increase of 35,126 new cases is expected by 2020 in Spain alone. The aim of this review is to study the relationship between obesity and the prognosis of prostate cancer, which is playing an increasingly important role in our health system.
Method
A systematic search in several databases (MEDLINE, Web of Science core collection and SCOPUS) was conducted. This search included only observational studies (cohort and case-control studies) from 2015 to
February 2020 on prostate cancer-specific mortality and one obesity marker (body mass index, BMI). The data extracted are the Hazard Ratio.
Results
8 studies (7 prospective cohorts and 1 retrospective cohort) were obtained from the search carried out for this review and their Hazard Ratio was analyzed. A positive association was obtained in 5 studies for a
BMI>27.5 kg/m² and BMI<22.5 kg/m². Paradoxically, one associated a protective association for a BMI<25 kg/m².
Conclusion
The literature reviewed indicates that obesity appears to be a bad prognostic factor in prostate cancer. However, there is not enough scientific evidence to validate this statement as no studies with conclusive
results were found. Therefore, more studies with other, more specific obesity factors are needed in order to conduct a more comprehensive analysis to confirm this association.
Keywords: obesity, body mass index, BMI, prostate cancer, specific mortality, prognosis.
INTRODUCTION
Prostate cancer (PC) is one of the most common cancers among men. According to data from the Spanish Society of Medical Oncology (SEOM, by its Spanish acronym), PC was the second most diagnosed in cancer in 2018, and the first in 2020 (1). Due to its prevalence, it is (along with other cancers) a public health problem. In terms of mortality, PC is the third in Spain, after lung and colorectal cancer. Despite this, it has a 5-year age-adjusted survival rate of 89.8% (observed: 78.9%) according to data from the Spanish Network of Cancer Registry (2008-2013) (2).
Its prognosis is influenced by several factors: morphological parameters of the tumor, histological type, location, perineural invasion, lymphovascular invasion, DNA ploidy, cell proliferation rate, p53 mutation, BCL2 gene, p27 gene, COX-2 overexpression, factor B and E-cadherin and whether it has an androgen receptor or not. (3). All these variables are influenced by diet, occupational factors, social factors, tobacco and alcohol, infectious diseases, hormonal stimulation, ethnicity and obesity (4). Obesity is beginning to play an increasingly important role in the development and prognosis of PC. When relating obesity to its etiological role, there does not seem to be a significant relationship in the literature with the risk of suffering PC (5). Its relationship with prognosis, however, is more doubtful. (6). Nonetheless, the body mass index (BMI), abdominal circumference and weight, which are markers of obesity, are used to decide on treatment and predict its response (7).
The aim of this review is to try to find a relationship between obesity and life expectancy of PC. For this purpose, BMI is taken as a marker of obesity and prostate cancer–specific mortality (PCSM) as a marker of life expectancy to further investigate this association, raise new hypotheses, and open new lines of study.
METHODOLOGY
This review was conducted following PRISMA guidelines (8). A search was performed using the following key words: 1) related to PC (‘prostate neoplasia’, ‘prostate cancer’, and ‘malignant prostate hyperplasia’), 2) combined with terms related to obesity (‘obesity’, ‘abdominal obesity’, ‘morbid obesity’, ‘body mass index’, and ‘weight’), 3) terms related to mortality (‘mortality’, ‘cause of death’, ‘survival rate’, and ‘quality of life’), 4) those related with prostate cancer recurrence were discarded (‘recurrence’, ‘repetition’, and ‘biochemical recurrence’). This search was performed in three different databases (MEDLINE, Web of Science Core Collection, and SCOPUS).
Only those articles from January 2015 to February 2020 were included, taking into account that they were original observational cohort and case/control articles, carried out in humans, and written in Spanish and/or English. A general review of titles and abstracts was then conducted by both authors (P.O. and J.J.P.). The exclusion criteria were: 1) articles dealing with crude death rate of PC and not with net death rate (PCSM), 2) that did not use BMI as marker of obesity, 3) in which weight loss was part of PC treatment, 4) in which only crude death rate was considered, 5) that considered obesity as a risk factor for the disease, 6) in which ill patients were compared to healthy patients, 7) that were either meta-analysis or reviews that resembled this one.
Data extraction
Using a standardized form made by the two authors (P.O. and J.J.P.), data were extracted from t: 1) the title, 2) year, 3) study population, 4) sample size, 5) follow-up period, 6) mortality data and BMI (kg/m2), 7) number of results, and 8) adjustment variables. Relative risk (RR), odds ratio (OR), and hazard ratio (HR) data, adjusted with a 95% confidence interval (CI) were extracted.
RESULTS
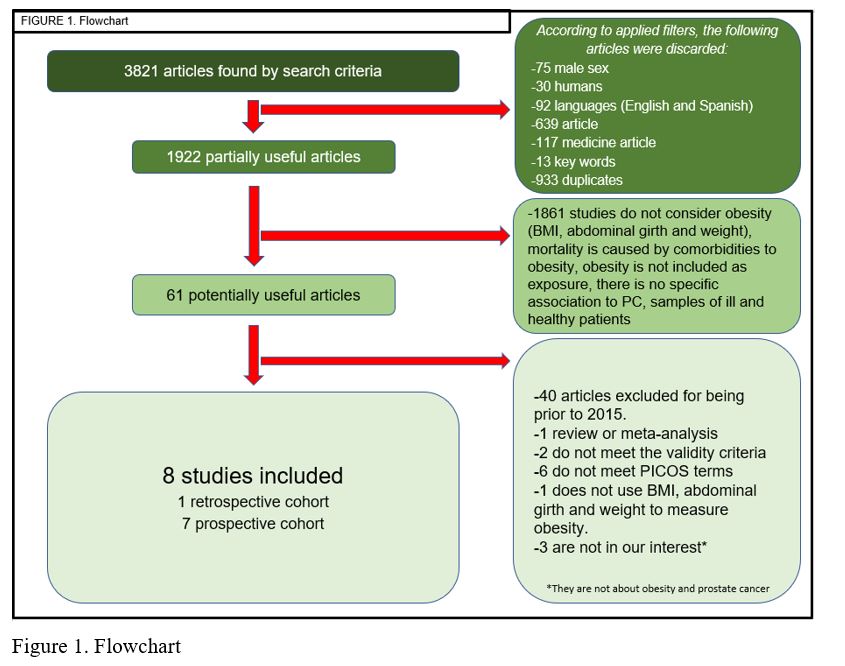
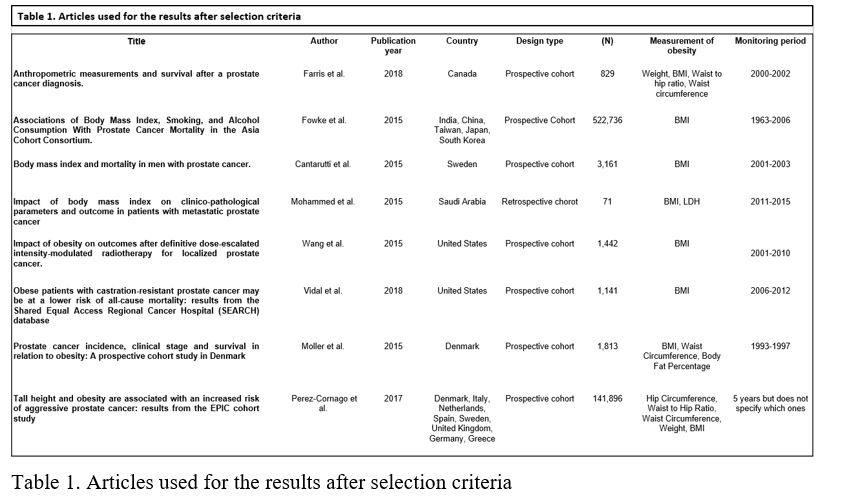
After the application of the previous method, 61 potentially useful articles were obtained. Of those, 40 were discarded since they were previous to 2015. Next, full texts were analyzed thoroughly, applying the same selection criteria. Eventually, 8 articles (Figure 1) were selected: 7 were prospective cohort studies (9-15), and 1 was a retrospective cohort study (16) (Table 1).
Only relevant results from the relationship between PCSM and obesity were included. The study by Moller et al. (14) considered as a control value BMI<20 kg/m2 (HR 1. Category 1 (C1) included men with PC whose BMI was between 20 and 30 kg/m2 in which HR was: 1.08 (95%CI 0.83-1.40) and Adjusted Hazard Ratio (aHR): 1,10 (95%CI 0.85-1.43). Category 2 (C2) included men who had BMI>30 kg/m2 with HR: 1.43 (95%CI 1.01-2.91) and aHR: 1.27 (95%CI 0.9-1.8).
The study by Farris et al. (9) classified patients in three categories according to BMI: 1) patients with PC and BMI<25 kg/m2 were used as control value, 2) C1 included those with BMI between 25-30 kg/m2 from which HR:0,84 (95%CI: 0.56-1.27) and aHR: 0.73 (95%CI: 0.48-1.11) were obtained, and 3) C2 included those with BMI>30 kg/m2 whose results were HR: 1,08 (95%CI: 0.71-1.66) and aHR: 0,97 (95%CI: 0.63-1.50). Vidal et al. (13) grouped patients into four different categories according to BMI: low weight for patients with BMI<21 kg/m2, being their HR:1.19 (95%CI: 0.71-1.97) and aHR: 1.14 (95%CI: 0.67-1.95); normal weight for patients who had BMI values between 21-24.9 kg/m2, which was taken as a control value; overweight for those who had BMI values between 25-29.9 kg/m2 with HR:1.20 (95%CI: 0.95-1.53) and aHR:1.17 (95%CI: 0.92-1.50); and obese for those with BMI>30 kg/m2 with HR: 1.18 (CI: 95% 0.92-1.50) and aHR: 1.11 (95%CI: 0.86-1.43).
Fowke et al. (10)divided patients into three groups: C1 included men with PC with BMI>25 kg/m2, obtaining a HR: 1.08 (CI 95% 0.85-1.36); those with BMI between 22.5-24.9 kg/m2 (HR 1) were used as control value); C2 included those with BMI between 20-22.4 kg/m2, with HR: 0.92 (95%CI: 0.75-1.13); and C3 included those with BMI<19.9 kg/m2, whose results were HR: 0.98 (95%CI: 0.78-1.23).
None of these studies showed a statistically significant association between obesity and PCSM, except Moller et al. (14), in which it did exist for patients with BMI>30 kg/m2, although it was the non-adjusted value.
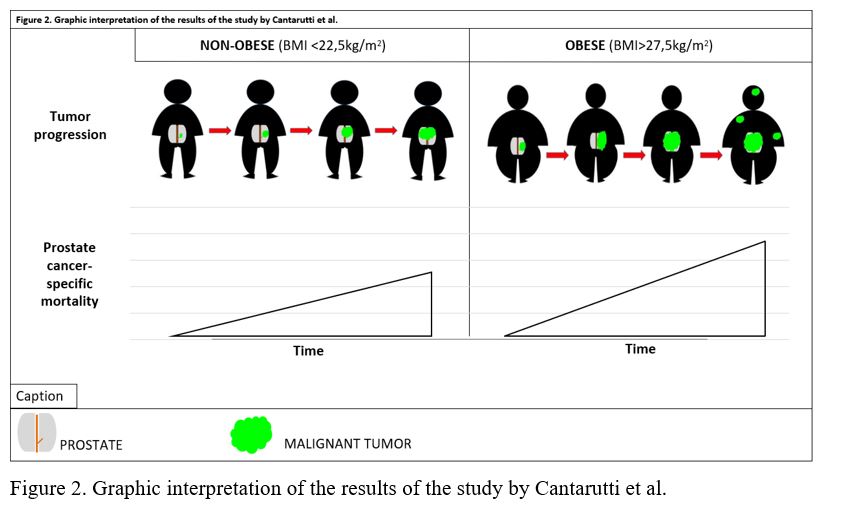
Cantarutti et al. (11) used as control value BMI between 22.5-25 kg/m2 (HR 1), C1 included patients with BMI<22.5 kg/m2 whose results were HR: 1.33 (95%CI: 1.02-1.74) and aHR: 1.31 (95%CI: 1.00-1.72); C2 included those with BMI 25<27.5 kg/m2 with HR: 1.01 (IC95% 0.96-1.43), and aHR: 1.05 (95%CI: 0.85-1.29); and C3 included those with BMI >27.5 kg/m2 whose results were HR: 1.17 (95%CI: 0.96-1.43) and aHR: 1.29 (95%CI: 1.05-1.53). A significant non-adjusted value for C1 subjects and a significant association for C3 subjects were observed (Figure 2).
Wang et al. (12) obtained the following results: sample population of 1,442 patients with localized PC, of which 284 had BMI <25 kg/m2; 688 BMI between 25-29.9 kg/m2; 326 BMI between 30-34.9 kg/m2; 92 BMI 35-39.9kg/m2, and 52 BMI>40 kg/m2. PCSM at 5 years was 0.8% (95%CI: 0.4%-1.6%). A positive association between the increase in the BMI and PCSM (HR: 1.15. 95%CI: 1.07-1.23) was also found when analyzing the data with a multivariate analysis.
The study population in the study by Pérez-Cornago et al. (15) included 141,896 men with PC, of which 931 of the recorded deaths were PC-related and a positive association with BMI was found (HR: 1.14 95%CI: 1.02-1.27). Mohammed et al. (16) obtained a sample of 71 patients with PC metastasis which were classified into two groups: group 1 (BMI <25 kg/m2 (N31)) and group 2 (BMI >25 kg/m2 (N40)). In the first group the PCSM was 7. In the second, it was 14. When associating BMI<25 kg/m2 with PCSM, a HR = 0.17 (95%CI: 0.04–0.70) was analyzed, showing a protective association for this group.
DISCUSSION
A range of possible answers have been found in the analyzed studies, some of which are contradictory. In some cases, the high BMI at the time of diagnosis and the increase in BMI during the disease is related to a higher PCSM (9, 11-12, 15). In contrast, other shows a protective association between these two variables (16).
Regarding the relation between BMI increase and PCSM increase, despite not finding a statistically significant association, this relation seems to be affected by other variables of obesity such as abdominal girth, specific biochemical markers (HDL, LDL, adiponectin) or height. Some of them seem to positively influence an increase in PCSM, such as height. Perez-Cornago et al. (15) associated a tall person with a normal BMI and high adipose tissue mass with a higher risk of PCSM. However, the inverse relation between height and BMI does not mean that tall people with a lower BMI have lower levels of adiposity. These data may lead to a false association between BMI and PCSM (9, 14). Yang et al. (17) did not find an association between BMI and PCSM. Nevertheless, when comparing this study with the one by Chalfin et al. (18), an association with a higher risk of aggressivity, progression and PCSM was observed. This revealed that obese men have a higher probability of suffering a more aggressive and higher relapse risk PC.
Along with the study by Chalfin et al., Wang et al. (12) also showed a positive association between BMI and relapse risk; PCSM; death rate and metastasis suffered by PC patients treated with radiotherapy. Reference is made to a multivariate analysis of age, androgen deprivation therapy and PSA pre-treatment as a log-transformed variable. Applying all these variables at the same time could lead to confusion. Therefore, data should be analyzed separately to avoid potential bias. Consequently, a new study model is needed, which takes into account BMI of the sample and the type of treatment depending on the patient and PC stage to determine the mechanisms involved in obesity and PCSM, and to determine whether or not an association exists and, if that is the case, what type.
Cantarutti et al. (11) found a correlation between a high PCSM and high BMI as compared to a normal BMI at the time of diagnosis. Their results also showed that a lower BMI also implied a higher PCSM although, in those cases, mortality from other causes also increased. These data are in line with those collected in the literature (19, 20).They suggest that different BMI ranges need to be further explored, i.e., to assess both the low and high ranges and their relationship to PCSM.
The study by Vidal et al. (13)showed that BMI is not associated with an increase in PCSM. However, when comparing these results with those of Halabi et al. (21), it they concluded that there is a protective factor between high BMI and PCSM. Two theories can explain this protective role. On the one hand, a higher BMI means less cachexia, in addition to providing protection against possible future cachexia (which would increase life expectancy of PC patients). On the other hand, obese men have increased estrogen formation caused by adipose tissue, and this hormone in turn inhibits the growth of PC (22). This idea is supported by Greenlee et al. (23), who compiled 22 clinical trials on the association between BMI and mortality of different types of cancer with their treatments. No association was found between BMI and the mortality of any type of cancer. However, it was observed that in specific cases of PC treated with androgen deprivation therapy, it was statistically significant that high BMI acted as a protective factor in relation to PCSM. These results shed new light on the protective role of adipose tissue and its endocrine function.
Regarding this protective role, the study by Dickerman et al. (24) on the association between visceral fat and advanced and fatal disease is also worth mentioning. They described that this association is stronger and statistically significant among the patients with a BMI <27 kg/m2 and weaker and non-significant among those with BMI ≥ 27 kg/m2. However, the CIs obtained, as well as evidence for heterogeneity of BMI were not statistically significant. This casts doubt on the validity of the results.
Also relevant is the study by Langlais et al. (25)on the influence of having a high BMI at the time of diagnosis in the erroneous staging of the tumor. This error may lie in making the diagnosis, since the tests carried out might be misinterpreted due to the patient’s obesity. This can lead to erroneous therapy and therefore, a worse prognosis of the disease.
Finally, the study Dickerman et al. (26) analyzed the increase in BMI from age 21to the time of PC diagnosis and found no association. . This means that lifetime weight gain did not influence the aggressiveness of PC. However, to prove the influence on this association, a pre-determined age range closer to the age of risk for PC should be studied.
Limitations of the study
A broader research should be conducted, considering grey literature, clinical trials with subgroup analysis working on mortality/survival based on BMI, and the possible existence of unpublished work. In addition, the range of years in the literature search should be extended, and other markers of obesity should be included to better assess the effect on prognosis of PCSM (16).
No statistical, sensitivity or validity analysis of the studies have been performed during the screening and selection process, as well as quality assessment of the studies in a systematic manner by the authors independently. Nor was an analysis performed according to the patient time included in the studies or the stage-aggressiveness of the PC they suffer.
CONCLUSION
The current literature seems to indicate that obesity is a poor prognostic factor in PC but there is not enough evidence to support this. Consequently, studies with more concrete and specific methodologies are needed as the detrimental effect of obesity in this type of cancer is still unclear. It would be convenient to consider other markers of obesity apart from BMI (HDL, LDL and adiponectin) to shed new light on the possible influence (or not) of these markers independently or jointly in the prognosis of PC.
STATEMENTS:
Acknowledgements
This paper is part of the Teaching Innovation Project coordinated between the Faculty of Medicine and the Faculty of Translation and Interpreting of the University of Granada (UGR), within the framework of the FIDO Plan 2018-2020 of the UGR (code 563).
Ethical concerns
This paper did not require the approval of any ethics committee.
Conflicts of interest
The authors of this paper declare no conflicts of interest.
Funding
No funding was received for the production of this paper.
REFERENCES
- Cifras del cáncer en España | SEOM – Día Mundial del Cáncer 2020 [Internet]. SEOM | Día Mundial del Cáncer 2020. [Last access: 7 March 2020]. Available at: https://seom.org/dmcancer/cifras-del-cancer/
- REDECAN – Supervivencia [Internet]. [Last access: 22 March 2020]. Available at: http://www.redecan.es/redecan.org/es/pagesup2013.html?id=210&title=estimaciones-de-la-incidencia-del-cancer-en-espana,-2019
- De Torres Ramírez I. Factores pronósticos y predictivos del carcinoma de próstata en la biopsia prostática. Actas Urológicas Españolas. 2007;31(9):1025-44.
- Montes de Oca L, Scorticati C. Cancer de Prostata. Editorial medica panamericana; 2014. 400 p.
- Daniell HW. A better prognosis for obese men with prostate cancer. Journal of Urology. enero de 1996;155(1):220-5.
- Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2011;4(4):486-501.
- Froehner M, Kellner A-E, Koch R, Baretton GB, Hakenberg OW, Wirth MP. A combined index to classify prognostic comorbidity in candidates for radical prostatectomy. Bmc Urology. 2014;14:28.
- Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin (Barc). 2010;135(11):507-11.
- Farris MS, Courneya KS, Kopciuk KA, McGregor SE, Friedenreich CM. Anthropometric measurements and survival after a prostate cancer diagnosis. British Journal of Cancer. 2018;118(4):607-10.
- Fowke JH, McLerran DF, Gupta PC, He J, Shu X-O, Ramadas K, et al. Associations of Body Mass Index, Smoking, and Alcohol Consumption With Prostate Cancer Mortality in the Asia Cohort Consortium. American Journal of Epidemiology. 2015;182(5):381-9.
- Cantarutti A, Bonn SE, Adami H-O, Gronberg H, Bellocco R, Balter K. Body mass index and mortality in men with prostate cancer. Prostate. 2015;75(11):1129-36.
- Wang LS, Murphy CT, Ruth K, Zaorsky NG, Smaldone MC, Sobczak ML, et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2015;121(17):3010-7.
- Vidal AC, Howard LE, de Hoedt A, Kane CJ, Terris MK, Aronson WJ, et al. Obese patients with castration-resistant prostate cancer may be at a lower risk of all-cause mortality: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Bju International. 2018;122(1):76-82.
- Moller H, Roswall N, Van Hemelrijck M, Larsen SB, Cuzick J, Holmberg L, et al. Prostate cancer incidence, clinical stage and survival in relation to obesity: A prospective cohort study in Denmark. International Journal of Cancer. 2015;136(8):1940-7.
- Perez-Cornago A, Appleby PN, Pischon T, Tsilidis KK, Tjonneland A, Olsen A, et al. Tall height and obesity are associated with an increased risk of aggressive prostate cancer: results from the EPIC cohort study. BMC Medicine. 2017;15:115.
- Mohammed AA, El-Tanni H, Ghanem HM, Farooq MU, El Saify AM, Al-Zahrani AS, et al. Impact of body mass index on clinico-pathological parameters and outcome in patients with metastatic prostate cancer. Journal of the Egyptian National Cancer Institute. 2015;27(3):155-9.
- Yang L, Drake BF, Colditz GA. Obesity and Other Cancers. Journal of Clinical Oncology. 2016;34(35):4231.
- Chalfin HJ, Lee SB, Jeong BC, Freedland SJ, Alai H, Feng Z, et al. Obesity and long-term survival after radical prostatectomy. J Urol. 2014;192(4):1100-4.
- Meyerhardt JA, Ma J, Courneya KS. Energetics in colorectal and prostate cancer. J Clin Oncol. 2010;28(26):4066-73.
- Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28(26):4052-7.
- Halabi S, Ou S-S, Vogelzang NJ, Small EJ. Inverse correlation between body mass index and clinical outcomes in men with advanced castration-recurrent prostate cancer. Cancer. 2007;110(7):1478-84.
- Montgomery B, Nelson PS, Vessella R, Kalhorn T, Hess D, Corey E. Estradiol suppresses tissue androgens and prostate cancer growth in castration resistant prostate cancer. BMC Cancer. 2010;10:244.
- Greenlee H, Unger JM, LeBlanc M, Ramsey S, Hershman DL. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiology Biomarkers & Prevention. 2017;26(1):21-9.
- Dickerman BA, Torfadottir JE, Valdimarsdottir UA, Giovannucci E, Wilson KM, Aspelund T, et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer. 2019;125(16):2877-85.
- Langlais CS, Cowan JE, Neuhaus J, Kenfield SA, van Blarigan EL, Broering JM, et al. Obesity at diagnosis and prostate cancer prognosis and recurrence risk following primary treatment by radical prostatectomy. Cancer Epidemiology Biomarkers and Prevention. 2019;28(11):1917-25.
- Dickerman BA, Ahearn TU, Giovannucci E, Stampfer MJ, Nguyen PL, Mucci LA, et al. Weight change, obesity and risk of prostate cancer progression among men with clinically localized prostate cancer. International Journal of Cancer. 2017;141(5):933-44.
AMU 2020. Volumen 2, Número 1
Fecha de envío:
16/03/2020
Fecha de aceptación:
06/04/2020
Fecha de publicación:
29/05/2020