Antonio Sánchez-Merino 1; Miguel Ángel Huerta-Martínez 2; Alexandru Ovidiu Zabava 3
1 Faculty of Medicine, University of Granada (UGR)
2 Department of Pharmacology and Federico Olóriz Institute of Neurosciences, Faculty of Medicine and Biomedical Research Centre (CIBM), University of Granada (UGR)
3 Institut für Biologie, Karl-Franzens Universität Graz | University of Granada (UGR)
Translated by:
Paula Trillo-Peña 4; Ana Castillo-González 4; Rubén García-Delgado 4; Virginia Sagarra-García 4; Antonio Jódar-González 4; María Rodríguez-Palomo 4; Olga Fenoll-Martínez 4
4 Faculty of Translation and Interpreting, University of Granada (UGR)
Introduction
COVID-19 is an example of a newly emerging, infectious disease with pandemic potential. Although there are numerous studies on this disease, the main focus now is on relating COVID-19 with possible long-term sequelae, as well as neurological manifestations. The aim of this study is to investigate the relationship between SARS-CoV-2 infection and the development of new-onset seizures, that is, in patients who had not been previously diagnosed with epilepsy.
Methods
A systematic search of articles and preprints was performed in three databases (MedLine, Scopus and Web of Science) between February 24 and March 7, 2021. The MeSH terms and keywords used in the search were: (“SARS-CoV-2” OR “COVID-19”) AND (“Seizures” OR “Status Epilepticus” OR “Electroencephalography” OR “EEG”) NOT (“Epilepsy”).
Results
Twenty-one studies were included 21 studies in the systematic review after screening. It was estimated that approximately 2.9 % of COVID-19 patients with neurological symptoms developed new-onset seizures and about 0.67% of the total number of COVID-19 patients developed new-onset seizures. The most common coexisting symptoms among these patients were fever, vomiting, cough and malaise. Antiepileptic treatment was key to the improvement of the health status of patients who developed new-onset seizures.
Conclusion
With the limited data available, it is currently impossible to establish a direct association between SARSCoV-2 infection and the development of new-onset seizures. The pathophysiologic mechanism causing the seizures cannot yet be determined either. However, it can be concluded that generally, these seizures are successfully reverted with antiepileptic treatment and patients usually respond favorably.
Keywords: COVID-19, SARS-CoV-2, neurological sequelae, seizures, status epilepticus.
Introduction
Infectious diseases are a global problem, especially due to the emergence of new potentially dangerous infectious agents (1). The betacoronavirus genus is an example of a family of emerging diseases with pandemic potential (2): severe acute respiratory syndrome (SARS-CoV) in 2002, Middle East respiratory syndrome (MERS-CoV) in 2012 (3,4), and the first SARS-CoV-2 cases in 2019 (5,6). SARS-CoV-2 was declared a pandemic by the World Health Organization on March 11, 2020.
Articles analyzing the clinical features of the first SARS-CoV-2 outbreak reported a high incidence of rather nonspecific symptoms such as fever, cough, respiratory distress or diarrhea (7-9), with no mention of neurological manifestations. Neurological manifestations were first evaluated by Mao et al. 2020(10), where they were estimated to appear in 36% of COVID-19 patients. The most common symptoms found were dizziness, headache, loss of taste and loss of smell. The first case of seizures associated with COVID-19 was also reported in this study. However, most later reviews reported diverse neurological alterations (headache, dizziness or altered level of consciousness), with a much lower prevalence compared to respiratory complications (11,12). For this reason, neurological manifestations were reported later.
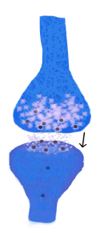
Regarding pathophysiology, the neuroinvasive pathway of SARS-CoV-2 is uncertain. Mainly, two hypothetical pathways have been suggested: neural dissemination (Figures 1 and 2) through the olfactory nerve and the cribriform plate, a pathway previously demonstrated for MERS-CoV and SARS-CoV in animals (13,14) and humans (15-17); and hematogenous dissemination (Figure 3) through cerebral circulation (18). Considering these possible pathways, neural invasion of SARS-CoV-2 may occur in a similar manner and be related to neurological symptoms (11).
Figure 1. Simplified representation of the axonal transport machinery. On the left, a neuron is depicted with cuts in the soma membrane in some parts of the axonal growth cone. This represents the hypothetical axonal transport of the virus.
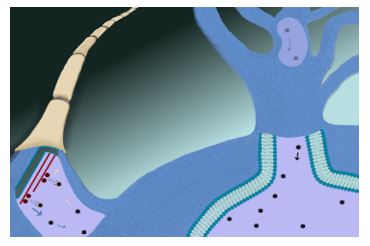
Figure 2. Neural dissemination. Presynaptic exocytosis and postsynaptic endocytosis of the virus are depicted.
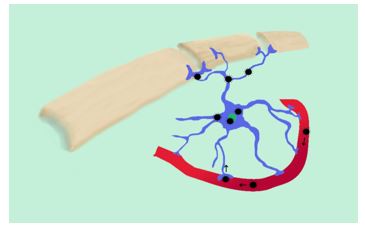
Figure 3. Presence of Coronavirus in astrocytes. Representation of an infected astrocyte and hematogenous dissemination.
Although there is an increasing number of articles on long-term, neurological sequelae in patients with COVID-19, there is still not much evidence on this regard and more studies are still needed to estimate them. Therefore, we performed a systematic review of the current literature on the topic, focusing on a very specific neurological manifestation: new-onset seizures. The reported cases of seizures caused by COVID-19 are few and the incidence is low. Still, they should not be dismissed, since they have an effective treatment and early detection could be of great clinical importance (19).
Therefore, the aim of this systematic review is to study the relationship between SARS-CoV-2infection and the development of new-onset seizures in patients who had not been previously diagnosed with epilepsy. The possible etiology of this manifestation, its outcome and potential associated comorbidities are also investigated.
Methods
A systematic review of the literature on COVID-19 and its neurological sequelae was performed. Special focus is placed on seizures in patients who had not been previously diagnosed with epilepsy. The PRISMA statement for reporting systematic reviews was followed as a guideline to develop this review (20).
Search strategy
A systematic search of articles and preprints was conducted in three databases (MedLine, Scopus and Web of Science) between February 24 and March 7, 2021. The MeSH terms and keywords used when searching for articles on seizures as a sequela of COVID-19were: (“SARS-CoV-2” OR “COVID-19”) AND (“Seizures” OR “Status Epilepticus” OR “Electroencephalography” OR “EEG”) NOT (“Epilepsy”).
Data management
The articles found after searching the databases were imported to Zotero (a free reference manager). After eliminating duplicates, the title and abstract of the remaining articles were read, eliminating those that were not related to the topic. The articles that were not relevant to the topic, those that did not mention seizures in patients with COVID-19 and those that only focused on neurological sequelae in general were also dismissed. Once the articles were screened, the reading process began to select the definitive articles for this systematic review. The selection criteria were the following:
- Original studies, cohort studies, case and control studies or case series published in English that provided information about patients not diagnosed with epilepsy who developed seizures in the context of COVID-19.
- Articles that provided sufficient information about patients, neurological symptoms (especially seizures), tests performed (imaging and laboratory tests), treatments and patients’ progress.
- Articles where the patients had tested positive for SARS-CoV-2 by any diagnostic method (PCR, serological tests or antigen test).
The exclusion criteria were:
- Systematic or narrative reviews, meta-analyses or letters to the editor.
- Studies that did not provide enough information about patients or studies where the information provided was not relevant for this review.
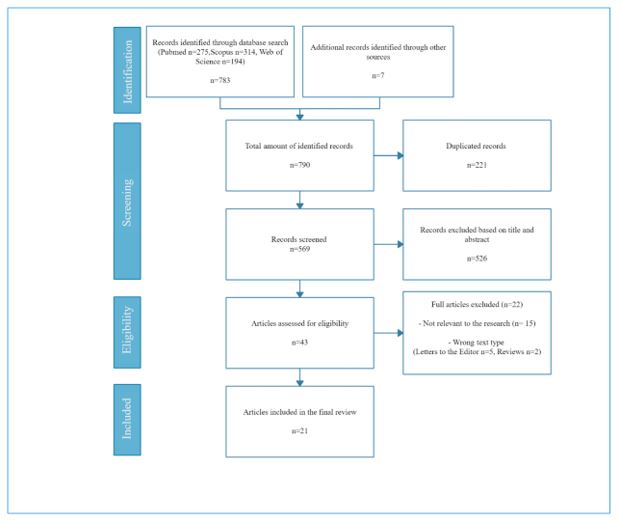
The choice of including or excluding the remaining articles was made by two of the three authors to avoid selection, performance or attrition bias. Once the choice had been made, the results were shared and any discrepancies were solved through dialogue. The selected articles were screened for other references that could provide original information about the topic of the study that may have been accidentally overlooked during the systematic search. The inclusion and exclusion criteria were applied to the relevant references. Figure 4 (PRISMA flowchart) summarizes the process described above.
Figure 4. Flowchart following PRISMA guidelines (20).
Lastly, two of the authors performed a bias assessment of all the observational studies included in the review using a modified version of the Newcastle-Ottawa Scale (NOS)to determine the quality of the studies. Discrepancies between the authors were solved through dialogue. As the NOS is not suitable for assessing case-control studies, these were excluded from the assessment because, according to the level of evidence of the JAMA network, these are the most biased (21).
Results
Of the 790 records identified, 21 studies were included in this systematic review, following the procedure described above. The selected articles are summarized in Tables 1, 2 and 3. Table 2 includes the values obtained on the modified NOS (22). The values were classified as unsatisfactory (0-3 points), satisfactory (4-5 points), good (6-7 points) and very good (8-9 points). Following these criteria, four articles were rated as satisfactory, three as good and one as very good. The detailed assessment of bias for each article according to the modified NOS can be found in the appendix (Table 1).
Table 1 summarizes the information obtained (23-35). Some of the most common symptoms among are fever, cough, vomiting and malaise. Most studies show that patients present comorbidities, although we found some exceptions. For example, in a study by Farsano et al. (26), a patient without previous history developed a seizure with clonic movements of the right arm. In another study by Suhail Hussain (31), a patient with hardly any symptoms experienced four episodes of generalized clonic-tonic seizures. Regarding the treatment, the efficacy of antiepileptic drugs is noteworthy. With the exception of four studies where patients worsened or even died (28, 30), studies showed that patients stopped having seizures and their health status improved until they were eventually discharged.
Tables 2 and 3 summarize the cohort observational studies (36-42). Thanks to the sample size, it was possible to estimate the frequency of new-onset seizures in COVID-19 patients by mathematical analysis. Thus, it was estimated that approximately 2.9% of patients with neurological symptoms derived from COVID-19 will develop new-onset seizures. The total proportion of COVID-19 patients that will develop new-onset seizures was also estimated, as shown in Table 3. It was estimated that 0.67% of COVID-19 patients will suffer from new-onset seizures. Table 2 explains the mathematical analysis performed.
Discussion
This systematic review estimated that less than one in ten patients with COVID-19 and neurological symptoms developed seizures, and that out of every 10,000 COVID-19 patients, 67 developed new-onset seizures. These data indicate that although neurological alterations are common among COVID-19 patients (43), this is not the case with seizures. In fact, paradoxically, these cases represent a relatively low proportion (between 1% and 26%) in contrast to other neurological manifestations (36, 38).
Patients that develop new-onset seizures associated with COVID-19 usually share a great number of comorbidities and suffer from severe COVID-19 symptoms. However, despite the severity, most seizures revert successfully with antiepileptic drug treatment (44). In addition, as shown by Hepburn et al., 2020 (33), patients with clinical signs of seizures or unexplained encephalopathy can benefit from electroencephalographic monitoring in addition to empiric antiepileptic treatment.
There are different hypotheses for the appearance of these seizures. On one hand, they might be associated with the pathophysiologic characteristics of severe cases of COVID-19. These cases present hypoxic encephalopathy, cardiovascular events and hypercytokinemia, which might be responsible for triggering these seizures (45). On the other hand, seizures might be caused by invasion of the nervous system by the virus, as suggested in different studies in which was detected in the CSF of COVID-19 patients that subsequently developed encephalitis (46). Lastly, the appearance of seizures in COVID-19 patients might be nothing but a mere coincidence and a matter of chance. This is not surprising considering the extremely low ratio of reported COVID-19 cases with seizures and the total amount of reported COVID-19 cases (47).
Regarding the strengths of this study, it is important to point out the adherence to the PRISMA system when elaborating this review. The exhaustive and comprehensive search carried out, the meticulous interpretation of all the studies included, as well as the assessment of potential bias are also worth highlighting. Moreover, the quality of the studies was determined using the NOS. Regarding the limitations of this study, it is possible that the search equation used and the use of only three databases and may have led to relevant studies being overlooked. The short follow-up time of observational studies may be another possible limitation of this study.
Conclusion
Although little is known about the sequelae of COVID-19, this review presents different perspectives and estimates on the incidence rates of new-onset seizures in COVID-19 patients. About 2.9 % of COVID-19 patients with neurological symptoms developed new-onset seizures and about 0.67% of the total number of COVID-19 patients developed new-onset seizures. However, with the limited data available, it is currently impossible to establish a direct association between SARS-CoV-2 infection and the development of new-onset seizures. The pathophysiologic mechanism behind the seizures cannot yet be established, but it can be concluded that antiepileptic drug treatment can successfully revert the seizures and that patients usually make good progress.
Statements
Acknowledgements
The authors of this paper would like to thank the involvement of the coordinating and teaching staff of the “Producción y traducción de artículos científicos biomédicos (III ed.)” and the “Traducción inversa de artículos científicos biomédicos (español-inglés)” courses, as well as the English translation team.
Conflicts of interest
The authors of this paper declare no conflicts of interest.
References
- Gao GF. From «A»IV to «Z»IKV: Attacks from Emerging and Re-emerging Pathogens. Cell. 2018;172(6):1157-9.
- de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-34.
- Anderson RM, Fraser C, Ghani AC, Donnelly CA, Riley S, Ferguson NM, et al. Epidemiology, transmission dynamics and control of SARS: the 2002-2003 epidemic. Philos Trans R Soc B Biol Sci. 2004;359(1447):1091-105.
- Mackay IM, Arden KE. MERS coronavirus: diagnostics, epidemiology and transmission. Virol J. 2015;12(1):222.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33.
- Coronavirus Disease (COVID-19) – events as they happen [Internet]. [citado 14 de marzo de 2021]. Disponibleen: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507-13.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497-506.
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683.
- Fathi M, Vakili K, Sayehmiri F, Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M, et al. The prognostic value of comorbidity for the severity of COVID-19: A systematic review and meta-analysis study. PLoS ONE. 2021;16(2): e0246190.
- Favas TT, Dev P, Chaurasia RN, Chakravarty K, Mishra R, Joshi D, et al. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. NeurolSci Off J Ital NeurolSoc Ital SocClinNeurophysiol. 2020;41(12):3437-70.
- Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264-75.
- K L, C W-L, S P, J Z, Ak J, Lr R, et al. Middle East Respiratory Syndrome Coronavirus Causes Multiple Organ Damage and Lethal Disease in Mice Transgenic for Human Dipeptidyl Peptidase 4. J Infect Dis. 2015;213(5):712-22.
- Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. AdvExp Med Biol. 2014;807:75-96.
- Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses. 2019;12(1).
- Veronese S, Sbarbati A. Chemosensory Systems in COVID-19: Evolution of Scientific Research. ACS ChemNeurosci. 2021;12(5):813-24.
- Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS ChemNeurosci. 2020;11(7):995-8.
- Vohora D, Jain S, Tripathi M, Potschka H. COVID-19 and seizures: Is there a link? Epilepsia. 2020;61(9):1840-53.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Instructions for Authors [Internet]. Chicago: American Medical Association (JAMA); 2021 [citado 14 de marzo de 2021]. Disponibleen https://jamanetwork.com/journals/jama/pages/instructions-for-authors#SecReviews.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa: The Ottawa Hospital Research Institute; c2021. [citado 14 de marzo de 2021] Disponibleen: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Chen W, Toprani S, Werbaneth K, Falco-Walter J. Status epilepticus and other EEG findings in patients with COVID-19: A case series. Seizure. 2020;81:198-200.
- Ashraf M, Sajed S. Seizures Related to Coronavirus Disease (COVID-19): Case Series and Literature Review. Cureus. 2020;12(7):e9378.
- Bhagat R, Kwiecinska B, Smith N, Peters M, Shafer C, Palade A, et al. New-Onset Seizure With Possible Limbic Encephalitis in a Patient With COVID-19 Infection: A Case Report and Review. J Investig Med High Impact Case Rep. 2021;9:2324709620986302.
- Fasano A, Cavallieri F, Canali E, Valzania F. First motor seizure as presenting symptom of SARS-CoV-2 infection. NeurolSci Off J Ital NeurolSoc Ital SocClinNeurophysiol. 2020;41(7):1651-3.
- Haddad S, Tayyar R, Risch L, Churchill G, Fares E, Choe M, et al. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020;21:e00814.
- Sohal S, Mansur M. COVID-19 Presenting with Seizures. IDCases. 2020;20:e00782.
- Hwang ST, Ballout AA, Mirza U, Sonti AN, Husain A, Kirsch C, et al. Acute Seizures Occurring in Association With SARS-CoV-2. Front Neurol. 2020;11:576329.
- Hamidi A, Sabayan B, Sorond F, Nemeth AJ, Borhani-Haghighi A. A Case of Covid-19 Respiratory Illness with Subsequent Seizure and Hemiparesis. Galen Med J. 2020;9:e1915.
- Hussain S, Vattoth S, Haroon KH, Muhammad A. A Case of Coronavirus Disease 2019 Presenting with Seizures Secondary to Cerebral Venous Sinus Thrombosis. Case Rep Neurol. 2020;12(2):260-5.
- Khan Z, Singh S, Foster A, Mazo J, Graciano-Mireles G, Kikkeri V. A 30-year-old male with COVID-19 presenting with seizures and leukoencephalopathy. Sage Open Med Case Rep. 2020;8:2050313X20977032.
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–8.
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55-8.
- Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus. 2020;12(3):e7352.
- Nalleballe K, Reddy Onteddu S, Sharma R, Dandu V, Brown A, Jasti M, et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020;88:71-4.
- Waters BL, Michalak AJ, Brigham D, Thakur KT, Boehme A, Claassen J, et al. Incidence of Electrographic Seizures in Patients With COVID-19. Front Neurol. 2021;12:614719.
- Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95(8):e1060-70.
- Mahammedi A, Saba L, Vagal A, Leali M, Rossi A, Gaskill M, et al. Imaging of Neurologic Disease in Hospitalized Patients with COVID-19: An Italian Multicenter Retrospective Observational Study. Radiology. 2020;297(2):E270-3.
- Kremer S, Lersy F, de Sèze J, Ferré J-C, Maamar A, Carsin-Nicol B, et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology. 2020;297(2):E242-51.
- Pinna P, Grewal P, Hall JP, Tavarez T, Dafer RM, Garg R, et al. Neurological manifestations and COVID-19: Experiences from a tertiary care center at the Frontline. J Neurol Sci. 2020;415:116969.
- Radmard S, Epstein SE, Roeder HJ, Michalak AJ, Shapiro SD, Boehme A, et al. Inpatient Neurology Consultations During the Onset of the SARS-CoV-2 New York City Pandemic: A Single Center Case Series. Front Neurol. 2020;11:805.
- Solomon T. Neurological infection with SARS-CoV-2 — the story so far. Nat Rev Neurol. 2021;17(2):65-6.
- Asadi-Pooya AA. Seizures associated with coronavirus infections. Seizure. 2020;79:49-52.
- Nikbakht F, Mohammadkhanizadeh A, Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. MultSclerRelatDisord. 2020;46:102535.
- Nwani PO, Nwosu MC, Nwosu MN. Epidemiology of Acute Symptomatic Seizures among Adult Medical Admissions. Epilepsy Res Treat. 2016;2016:4718372.
- Goleva SB, Lake AM, Torstenson ES, Haas KF, Davis LK. Epidemiology of Functional Seizures Among Adults Treated at a University Hospital. JAMA Netw Open. 2020;3(12):e2027920.
AMU 2021. Volumen 3, Número 1
Fecha de envío:
14/03/2021
Fecha de aceptación:
04/04/2021
Fecha de publicación:
31/05/2021