Ariana Guiomar-Córdova 1; Magdalena Bustos-Romano 1; Daniel Sánchez-Correa 1
1 Faculty of Science (Biology), University of Granada (UGR)
Translated by:
Sofía Antequera-Manzano 2; Beatriz Arellano-Romero 2; Samuel De la Torre-Galán 2; Carmen Gámez-Salazar 2; Clara Sofía GilOrtega-Barahona 2; Amanda Inés Hernández-García 2; Francisco José Villena-Rodríguez 2
2 Faculty of Translation and Interpreting, University of Granada (UGR)
This non-systematic review gathers data on neurological sequelae of COVID-19 patients aged 18 to 65 years. Scientific evidence shows that the main SARS-CoV-2 entry occurs through the ACE2 receptor, which is present in numerous cells of the organism. The virus penetrates the blood-brain barrier through vascular endothelial cells and immune cells; via the olfactory bulb and optic nerve channels; and across trans-synaptic transmission. In the central nervous system, the cytokine storm culminates in oxygen deprivation to neuronal cells. In the long term, this may lead to neurodegenerative diseases such as encephalopathy. Preliminary studies on treatment suggest a symptomatic approach proposing some pharmaceutical drugs such as adamantane. Therefore, some COVID-19 patients could experience cognitive sequelae, which could be related to the inflammation level produced by the infection.
Keywords: COVID-19, cognition, ACE2, spike protein, neuronal injury, neurological sequelae.
Introduction
The SARS-CoV-2 agent (1,2) was identified in December 2019 at the Huanan seafood market in Wuhan, Hubei (China). This virus caused atypical pneumonia of unknown origin and muscle aches, among other symptoms (2). Since then, the number of cases has increased significantly around the world (1). The WHO declared the situation to be a pandemic on March 11, 2020 (2). As of March 28, 2021, a total of 126,607,206 positive cases and 2,776,023 deaths were confirmed worldwide, according to data provided by Johns Hopkins University (3).
SARS-CoV-2 is a new betacoronavirus responsible for COVID-19 (4). The median incubation period is approximately two days, although it can vary from one to four days (5). The most common symptomatology produced by this microorganism includes fever, cough, fatigue, headache, anosmia, hyposmia, ageusia, dyspnea, and chest pain, among others (6–8). The second most common manifestation is altered mental status (9). Cerebrovascular injury and impaired consciousness developed within a mean period of 8 to 10 days after admission, while the rest of neurological manifestations occurred within a mean period of 1 to 2 days after admission. Neurological manifestations were more frequent in severe COVID-19 infected patients than in those with non-severe infection (10). The most common deficiencies are altered mental statuses such as delirium (13), severe cognitive, consciousness and personality disorder, and an alteration of the peripheral nervous system (9). Moreover, SARS-CoV-2 has been related to more severe symptoms. Examples include dysarthria, dystonia, seizures, confusion, and even death (11,12).
This non-systematic review aims to gather data provided by scientific evidence on the neurological sequelae of COVID-19 patients. In addition, this paper studies the possible mechanisms of SARS-CoV-2 entry into both cells and the organism through diffusion pathways to the central nervous system (CNS), and whether any treatment is currently available.
Methods
This review collected the studies found using the search equation ‘cognitive sequelae covid’ in the Medline database. The selection criteria were patients aged 18 to 65 years. Researchers have studied the cognitive sequelae of a post-COVID patient compared to the normal cognitive response in patients who have not been infected. This review has collected all studies published in 2020 related to this topic.
SARS-CoV-2 entry into human cells through the ACE2 receptor
Many chronic degenerative diseases could be the consequence of neuronal injury caused by COVID-19. Despite the potential direct or indirect effects of the disease, COVID-19 may permanently damage the CNS (14). One of the most vulnerable areas to ischemic injury by COVID-19 and of vital importance to cognitive function is cerebral white matter. The cumulative destructive effect of multifocal cerebral ischemia or hemorrhage together with the complications that arise after the disease, such as endothelial and blood-brain barrier (BBB) dysfunction and upregulation of pro-inflammatory cytokines within the brain, may considerably increase the risk of chronic brain injury (15). In addition, a potential connection is established between cognitive impairment and neurodegeneration and adult respiratory distress syndrome (ARDS) (16). A higher report incidence of this connection was observed in elderly patients or those with COVID-19, or those with previous pathologies such as hypertension, diabetes or underlying cerebrovascular disease. However, young adults with COVID-19 also presented this incidence (15).
The spike protein of SARS-CoV-2 together with the ACE2 helps the virus enter into the cell and allows it to infect it. The distribution of the expression of the ACE2 receptor in different locations in animal and human brain tissues is widely expressed in neurons, astrocytes, and oligodendrocytes throughout the brain (14) (Figure 1). Post-mortem studies have shown three major findings:
- ACE2 receptor, the main receptor of SARS-CoV-2, is widely expressed in brain microvascular endothelial cells, in pericytes throughout the body and in the brain (14,15).
- The spike protein can directly damage the integrity of the BBB to varying severity levels (14).
- The spike protein induces the inflammatory response of microvascular endothelial cells, which leads to BBB dysfunction (14).
Figure 1. Schematic illustration of the binding of SARS-CoV-2 spike protein to the ACE2 receptor present in the central nervous system.
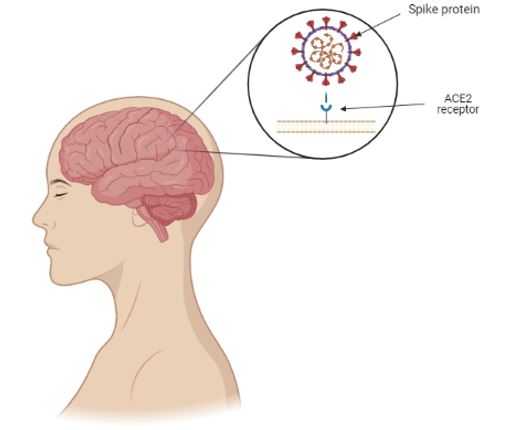
The virus binds to the ACE2 receptor in two different ways:
- Via the vascular endothelial cells. Vascular endothelial cells regulate BBB permeability through tight junctions. These junctions present an extensive expression of the ACE2 receptor, which provides the molecular mechanism by which SARS-CoV-2 enters the BBB and invades the brain. The virus binds to the ACE2 receptor and enters the vascular endothelial cells by endocytosis and exocytosis, thus achieving cell-to-cell transmission. However, the virus does not replicate during transendothelial cell transfer. Viral replication delays until the virus reaches the target cells, such as neurons or glial cells, and binds to the ACE2 receptor before replication starts (14).
- Via the immune cells. Immune cells work as a bridge for SARS-CoV-2 to cross the BBB (17) using the Trojan horse mechanism. It requires two conditions: firstly, immune cells that express ACE2; and secondly, circumstances in which the virus does not replicate. Furthermore, the pathogen can enter macrophages cytoplasm by binding to the receptor on the surface using the ‘Trojan horse’ mechanism (14).
Mechanisms of entry into the organism
The respiratory tract is the main mechanism for SARS-CoV-2 to enter into the organism. This leads to pneumonia, which may be followed by fibrosis and chronic impairment of lung function. The infection by this pathogen affects the CNS and the peripheral nervous system (PNS) damaging both neurons and glial cells directly or indirectly. This may cause neuropathologies which could lead to long-term sequelae. Although virus transport via the olfactory bulb, associated with anosmia, is a potential route for neuroinvasion, the cerebral vasculature plays a more important role in entry of the virus into the CNS (15). The alteration of the BBB by SARS-CoV-2 and its entry into the brain support the formation of fatal microthrombi and even the occurrence of encephalitis (14). This association supports plausible hypotheses on the occurrence of long-term neurological sequelae. In addition, to cross the BBB, SARS-CoV-2 may enter the brain by:
– Optic nerve channel and olfactory bulb (18)
– Trans-synaptic transmission (19)
– Vascular endothelial cells (14)
The olfactory nerve contains both sustentacular cells, which maintain the integrity of the sense of smell, and stem cells, located in the olfactory epithelium. These cells express wild-type ACE2 and transmembrane serine protease 2 (TMPRSS2), which could merge SARS-CoV-2 to the olfactory bulb and the sustentacular cells, followed by travel into the brain (14).
SARS-CoV-2 enters the central nervous system through two different routes: the olfactory route and the blood-brain barrier route. The virus can migrate into the CNS by endocytosis, due to its increased vascular permeability, which is induced by pro-inflammatory cytokines and an indirect transfer via the ‘Trojan horse’ mechanism. After binding to its membrane receptor, ACE2, the pathogen will be engulfed into neuronal cytosol and will connect with the angiotensin-converting enzyme in the cytosol. Both the virus and the ‘cytokine storm’ can destroy the myelin sheath, resulting in acute and chronic neuropathology (14) (Figure 2).
Figure 2. Schematic illustration of the main mechanisms of SARS-CoV-2 entry into the central nervous system.
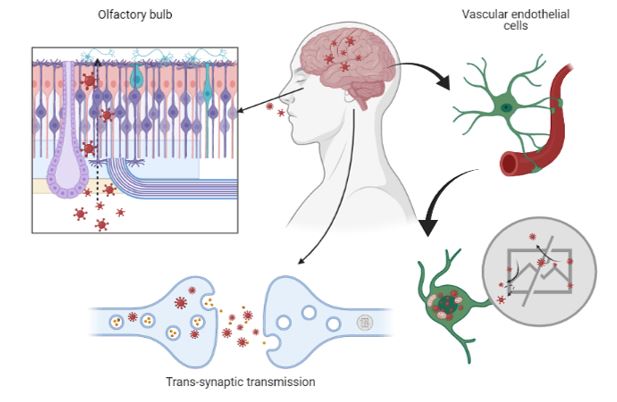
Optic nerve channel and olfactory bulb
The pro-inflammatory cytokines and the C-reactive protein (CRP) played a significant role in the development of SARS manifestation. SARS-CoV-2 particles are spread in the respiratory mucosa and infect other cells, inducing changes in peripheral immune cells. This elicits a cascade of immune responses, which results in a detrimental production of cytokines as this inflammation is directly linked to cognitive dysfunction (16).
Post-mortem studies on the cerebral pathology of COVID-19 patients and the use of an advanced 3D microfluidic model of the BBB identified that ACE2 receptor and the spike protein are expressed in brain microvascular cells. This protein can undermine the integrity of the BBB in different ways and induce inflammatory responses of microvascular cells, changing the function of the BBB. These findings support that the SARS-CoV-2 can alter BBB. Therefore, the virus can enter the brain and support the occurrence of neurological symptoms, the formation of fatal microthrombi, and even the occurrence of encephalitis. This supports the potential basis for the occurrence of long-term neurological sequelae (14).
When SARS-CoV-2 enters the CNS through the olfactory bulb, anosmia is triggered, causing neurodegenerative diseases due to loss of neurotransmitter induced by neuronal death. After the entry into the organism, viral replication may begin, releasing pro-inflammatory proteins. Triggered by oxidative mediators, loss of dopamine (DA) neurons or the aggregation of amyloid fibrils may result from the entry of the virus (18).
Trans-synaptic transmission
When SARS-CoV-2 binds to the ACE2 receptor, it travels in an axonal manner to reach the CNS. Once reached the synaptic cleft, membrane coating-protected exocytosis, endocytosis, and vesicle transport lead to trans-synaptic transmission of the virus from one neuron to another and from neuron to satellite cells. Furthermore, the rapid intracellular axonal transport provides a structural basis for further virus transmission (14).
Effects on the nervous system
No matter what method of invasion SARS-CoV-2 uses, when it reaches its destination, it replicates and uses its viral mechanisms to cause cell death or functional impairment. Rapid viral replication, direct cell injury, immune system activation, and inflammatory mediators (including cytokines) cause COVID-19 severe symptoms and may explain SARS-CoV-2 infection long-term sequelae. The increase of inflammatory mediators, the so-called cytokine storm, may explain the multiple organ injury found in some COVID-19 patients and may also explain the effects of SARS-CoV-2 on the CNS. This pro-inflammatory cytokine storm increases vascular permeability. Furthermore, this may trigger abnormal blood coagulation and multiple organ failure. These cytokines may also increase microvascular permeability within the CNS, facilitating the virus entry through the BBB. The ‘cytokine storm’ may also promote the formation of thrombi by activating the coagulation system (14).
After SARS-CoV-2 infection, brain injury is caused due to autoimmunity. Furthermore, this autoimmune attack is known to cause encephalopathies, with psychotic or neurological symptoms. The brain is protected by the BBB, which makes it particularly vulnerable to an autoimmune attack. However, this is not particularly frequent among COVID-19 patients.
Pneumonia and ischemia are two of SARS-CoV-2 infection main consequences, and they lead to severe and prolonged hypoxia, which affects the brain causing acute negative effects in the most severe cases. These negative effects are associated with:
– Arterial oxygen reduction, which causes impairments of neuronal activity
– Neuronal oxidation caused by hypoxia
The inflammation accompanying COVID-19 increases levels of fibronectin, which facilitates clot formation. Moreover, a large number of COVID patients demonstrated these complications. Thus, Covid-19 patients are more likely to develop neurodegenerative diseases (19) (Figure 3).
Figure 3. Schematic illustration of the damage potentially produced by SARS-CoV-2 in the central nervous system.
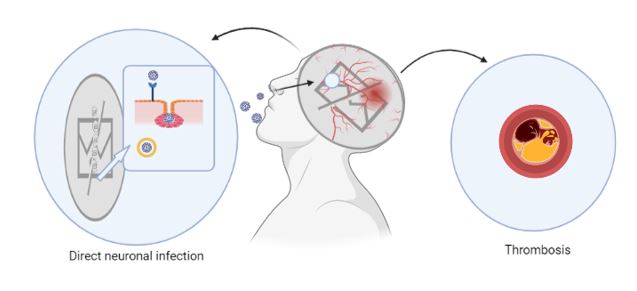
Long-term sequelae
Coronaviruses may persist in CNS resident cells and can be cofactors associated with the development of long-term neurological expressions in genetically predisposed individuals. Several coronaviruses have been identified by serological tests in a wide range of neurological pathologies, such as Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and optic neuritis. Coronaviruses 229E, 293, and OC43 have been isolated from the cerebrospinal fluid and brain of patients with multiple sclerosis. A substantially higher prevalence of OC43 coronavirus has been reported in the brains of patients with multiple sclerosis in comparison to those in a control group. Furthermore, the immune response after the infection may provoke multiple sclerosis flare-ups in susceptible individuals (20).
Since some effects of SARS-CoV-2 may appear many months or years after the infection, it would be advisable to monitor patients who have suffered COVID-19. The cytokine storm caused by infection may lead to small eventual cerebrovascular accidents without provoking noticeable neurological deficiencies. Once a patient overcomes COVID-19, they may experience memory and attention problems or slow processing speed. Therefore, patients are advised to seek advice from a neurologist or undergo neurocognitive tests from six to eight months after medical discharge if they are still experiencing cognitive disorders, slow processing of information or attention deficit (14).
Treatment and prevention
Although ACE2 receptors have been found in the ocular organs, ocular damage associated with a SARS-CoV-2 infection has not been reported. However, it is advisable to take preventive eye protection measures when in contact withSARS-CoV-2 infected patients to prevent potential spreads of COVID-19 via an ocular route (10).
Individuals with vascular risk, such as obesity, hypertension or diabetes, are prone to suffer more from the sequelae of a SARS-CoV-2 infection in comparison to any healthy individual. It is therefore advisable to improve the quality of life in the general population, thereby increasing patients’ likelihood of a more favorable recovery (4).
The research by Rejdak and Grieb (21) may suggest that adamantane has an antiviral protective effect. If this is proven, SARS-CoV-2 infection and its clinical neurological sequelae could be slowed by the consumption of these drugs, which are commonly employed as a causal treatment to mitigate the symptoms of Parkinson’s disease and cognitive dysfunction. Adamantane’s hydrocarbon structure may affect the viroporin channel, responsible for release of RNA-viruses (21). This could interfere with the release of SARS-CoV-2 from infected cells. Therefore, adamantane dispensation has been proposed as a potential treatment against COVID-19 for the most vulnerable patients.
Conclusion
The studies included in this review suggest that COVID-19 may affect cognition in some patients, and that this sequela could be related to the inflammation level produced by the infection. The SARS-CoV-2 viral spike protein can bind to the ACE2 cell receptor, present on the surface of multiple cells throughout the body. Thus, the virus enters through three possible pathways: via the olfactory bulb, through trans-synaptic diffusion or by vascular, or immune endothelial cells.
Certain cerebrovascular accidents that are predisposing to the development of neurodegenerative diseases, such as Parkinson’s disease or Alzheimer’s disease can emerge due to SARS-CoV-2 infection. Scientists have not developed effective treatments for these sequelae yet, but they have tested other pharmaceutical drugs such as adamantanes. However, specialists recommend seeing a neurologist six to eight months after suffering the disease for medical follow-up.
The main limitation of this study is the limited bibliography on this subject. Therefore, it is necessary to propose new studies in which patients are more closely observed to learn the characteristics of neurodegenerative sequelae.
Statements
Acknowledgements
The authors of this paper appreciate the involvement of the coordinating and teaching staff of the “Producción y traducción de artículos científicos biomédicos (III ed.)” and the “Traducción inversa de artículos científicos biomédicos (español-inglés)” courses, especially Pablo Redruello Guerrero for his dedication and mentorship, as well as the English translation team. The figures are of our creation and have been designed thanks to BioRender (22).
Conflicts of interest
The authors of this paper declare no conflicts of interest.
References
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet Lond Engl. 2020;395(10224):565-74.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507-13.
- Coronavirus COVID-19 (2019-nCoV) [Internet]. 2021 [cited 2021 Mar 8]. Available at: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. J Alzheimers Dis JAD. 2020;76(1):3-19.
- Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: A prospective contact-tracing study. J Infect. 2020;80(6):e1-13.
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20.
- Rivera-Izquierdo M, Valero-Ubierna M del C, R-delAmo JL, Fernández-García MÁ, Martínez-Diz S, Tahery-Mahmoud A, et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: A retrospective observational study. PLOS ONE. 2020;15(6):e0235107.
- Rivera-Izquierdo M, Valero-Ubierna MDC, Martínez-Diz S, Fernández-García MÁ, Martín-Romero DT, Maldonado-Rodríguez F, Sánchez-Pérez MR, Martín-de los Reyes LM, Martínez-Ruiz V, Lardelli-Claret P, Jiménez-Mejías E. Clinical Factors, Preventive Behaviours and Temporal Outcomes Associated with COVID-19 Infection in Health Professionals at a Spanish Hospital. Int J Environ Res Public Health. 2020;17(12):4305.
- Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-82.
- Lai C-C, Ko W-C, Lee P-I, Jean S-S, Hsueh P-R. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents. 2020;56(2):106024.
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767-83.
- Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(7):1549.e3-1549.e7.
- Mcloughlin BC, Miles A, Webb TE, Knopp P, Eyres C, Fabbri A, et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020; 11: 857–862
- Wang F, Kream RM, Stefano GB. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e928996-1-e928996-10.
- Miners S, Kehoe PG, Love S. Cognitive impact of COVID-19: looking beyond the short term. Alzheimers Res Ther. 2020;12(1):170.
- Zhou H, Lu S, Chen J, Wei N, Wang D, Lyu H, et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98-102.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762-8.
- Mahalaxmi I, Kaavya J, Mohana Devi S, Balachandar V. COVID-19 and olfactory dysfunction: A possible associative approach towards neurodegenerative diseases. J Cell Physiol. 2021;236(2):763-770.
- Verkhratsky A, Li Q, Melino S, Melino G, Shi Y. Can COVID-19 pandemic boost the epidemic of neurodegenerative diseases? Biol Direct. 2020;15(1):28.
- Carod-Artal FJ. Complicaciones neurológicas por coronavirus y COVID-19. Rev Neurol 2020;70 (09):311-322
- Rejdak K, Grieb P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Mult Scler Relat Disord. 2020;42:102163.
- BioRender [Internet]. 2021 [cited 2021 Mar 29]. Available at: https://app.biorender.com/
AMU 2021. Volumen 3, Número 1
Fecha de envío:
14/03/2021
Fecha de aceptación:
04/04/2021
Fecha de publicación:
31/05/2021