Celia Gómez-Gordo¹ , María Garzón-Polanco¹ , Tatiana Fokina¹.
1. Facultad de Medicina, Universidad de Granada (UGR).
TRANSLATED BY:
Pablo Sánchez-Ayuso², Laura Jiménez-Rivera², Ángel Peinado-Castillo², Pilar Torres-Jiménez², Miguel David Corado-
Palomo², Álvaro López-González².
2. Faculty of Translation and Interpreting, University of Granada (UGR).
Lyme disease (LD) is a zoonosis caused by bacteria of the genus Borrelia. LD is one of the most frequent diseases transmitted by ticks, being the most common in Europe and the United States. The geographical distribution of Borrelia is the reason why this is one of the most common borreliosis, which is also related to the habitat of its vectors (mostly the genus Ixodes). However, Borrelia is not the sole pathogen of this vector. As a consequence, some regions of northern Spain are considered to be borreliosis endemic areas, particularly of LD, due to the biogeochemical conditions of the area. Nevertheless, this zoonosis is widely spread throughout the country, due to the ubiquitous presence of mammals and other vertebrates carrying the vector (hosts). Although LD is very frequent across the Iberian Peninsula, the heterogeneity of clinical manifestations and vectors complicates its detection. Consequently, diagnosis is delayed and the disease develops, which leads to clinical complications.
Keywords: Lyme disease, borreliosis, Borrelia, Ixodes.
INTRODUCTION
Lyme disease (LD) is an infection that mainly affects animals, but can also be transmitted to humans (i.e. a zoonosis). This disease is caused by bacteria of the genus Borrelia (1), which belong to the phylum Spirochaetes (2). In Spain, the predominant species are B. burgdorferi and B. garinii, whose infections cause similar symptoms (3-5).
LD is transmitted by inoculation of the pathogen Borrelia through the chelicerae of the tick, which are appendages of the genus Ixodes mouthparts. These chelicerae cause a lesion in the epidermis of the host and, as a result, the hypostome is pulled into the skin, allowing its attachment. This leads to the infection of the host due to the pathogens carried by the tick (6). In Spain, the main vector of LD is Ixodes ricinus, which carries Borrelia burgdorferi and other species of this genus (4). The type of pathogen causing the infection is related to the biotic and abiotic conditions of the geographical area being studied (7). Therefore, it is essential to know both the climatic conditions and the type of vertebrates living in a region in order to identify the agent of the infection. Large vertebrates, including livestock, are the ideal hosts for most tick species. Furthermore, the movements and migrations of livestock are one of the factors that enables the spread of borrelioses in neighboring areas (7).
Although LD presents a wide variety of clinical manifestations, fever of unknown origin is not usual in this type of infections in a pediatric population (4). In fact, it has been reported that most people with borreliosis do not show fever as a symptom, unlike infections caused by other types of pathogens that are also transmitted by ticks (4). In the case of an atypical clinical picture, various types of borreliosis should be considered, even in the absence of fever in children (8). Since there is an under-diagnosis of LD, the presence of risk factors makes it necessary to perform a screening for the disease (3). This zoonosis is frequent among the pediatric population, with a peak incidence for children between 3 and 12 years old, which implies the need for an active diagnosis (4). This literature review collects data from a variety of sources in order to evaluate whether a correct differential diagnosis is performed in the presence or absence of risk factors for LD among people susceptible of being infected by pathogens of the genus Borrelia.
LYME DISEASE PREVALENCE AND INCIDENCE
The incidence of Lyme borreliosis in Spain is estimated at 0.25 cases per 100,000 inhabitants/year (3). Men are the most affected by this disease (3, 4), as they are more engaged in agricultural activities than women (3). At the same time, the incidence rises in high rainfall and coppice areas, since they favor the proliferation of hosts that can be colonized by ticks (4). Climatic factors are also important in pathogen transmission regarding its connection with the size of Ixodes colonies (4). Thus, more tick will live in areas with the most favorable factors. This explains the significant difference in incidence found between Asturias (maximum incidence) and the rest of autonomous communities, with the exception of Ceuta, where the rate is also higher than the Spanish average(3). Nonetheless, a study conducted on pediatric population in Galicia showed an average incidence of 5.5 cases per 100,000 inhabitants/year (4). In terms of incidence throughout the year, a higher infection rate of Borrelia is reported during the warmest months, between June and October (3,4). The increase on the number of ticks in these months is associated with both the favorable climate for their breeding and the rise in the amount of possible vertebrate hosts (7). In spite of this, due to the small size of Ixodes (Figure 1) (1,4), the tick bite is painless and less than half of the patients remembers being bitten (3,4,9). Consequently, it is estimated that the LD prevalence data in Spain are underrated due to the limitation of its sources, such as the Spanish Minimum Basic Data Set (MBDS). This data set exclusively gathers information of inpatient treatments and excludes minor cases treated by primary health care services, and those without any clinical contact. This information strongly depends on the quality of the medical report. Regardless of its limitation, the MBDS allows the estimation of prevalence in large populations (2, 10).

ETIOLOGICAL VARIABILITY OF VECTORS
Pathogen transmission in a certain geographic area will depend on the tick population of that specific region. There are several tick species, including Ixodes ricinus, one of the prevailing species in Europe (10). There is evidence that Ixodes ricinus can be colonized by different Borrelia species (Figure 2) such as B. burgdorferi, B. garinii, B. miyamotoi, B. afzelii, and B. lusitaniae (10).
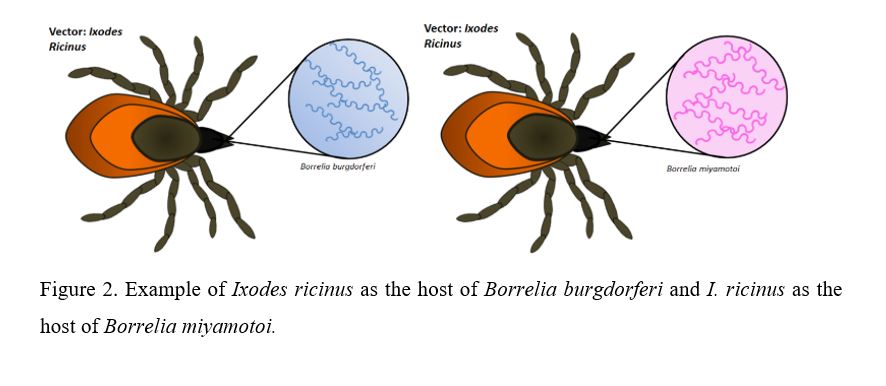
Biotic and abiotic factors of a specific area have to be considered in order to study the connection between types of Borrelia and infection prevalence. It has been proved that the relationship of ticks within their habitat mainly depends on climate and the diversity of vertebrates they can colonize. As a consequence, the greater the variety of vertebrates, the more colonies of Ixodes will live in that specific region (7). Furthermore, studies reveal that the presence of large mammals living in nature reserves enables tick population to increase (9). These conditions lead to a higher incidence rate of LD in rural areas with high diversity of host vertebrates during the warmest period of the year (3). In this respect, the northern regions of Spain are of great interest in terms of studying the prevalence of borreliosis in present mammals, and therefore a possible increase of tick population and human infection.
The relationship between hosts and ticks (interspecies relationship) is essential in order to understand pathogen transmission. In contrast, migration of vertebrates among different areas may explain the variability of species of genus Ixodes within a certain region. Thus, the migration of vertebrates which carry a particular species of Borrelia to an environmentally optimal region may allow this particular species to proliferate and develop in areas where they were not previously endemic (7).
The same species of vertebrates can be colonized by different types of ticks, which in turn can be hosts to different species of Borrelia. In fact, B. burgdorferi has been found not only in samples of Ixodes ricinus, but also in Haemaphysalis concinna, Haemaphysalis punctata, Rhipicephalus bursa and Dermacentor reticulatus (9). This can be explained by the fact that some Borrelia present a higher infectivity rate than others (7).
These interspecies relationships illustrate the diversity of pathogens which cause LD (7). According to a study which used network analysis, the amount of ticks and pathogen transmission could be limited by insufficient availability of vertebrates (7).
GEOGRAPHICAL DISTRIBUTION AND CLIMATIC CHARACTERISTICS
Geography plays an important role in the distribution of this disease. Thus, eastern and northern regions of Spain are endemic zones of the vector, and consequently, of Borrelia (11). Rural and mountain areas are highly susceptible for three reasons: contact with mammals is more frequent, there is more vegetation, and the climate is appropriate for Ixodes to spread (11).
A greater number of diagnoses has been reported between June and October, as there is a time lag of some months since patients remember being in contact with ticks and the appearance of clinical manifestations. Regarding these conclusions, a higher prevalence of tick infestation in animals between May and July has been observed (11).
HOST CHARACTERISTICS
No statistically significant differences regarding sex in the northeast area of Spain have been observed in the literature (11). This stands in contradiction to other data from Spain, which shows that it is more frequent among men (3). Hence, the geographical distribution is an important factor in determining the prevalence of LD. Significant differences have been found with regard to age, being the incidence of LD higher in people over 65 (11). Nevertheless, other studies report another peak incidence among children aged between 3 and 12 (4).
CLINICAL PRESENTATION AND TREATMENT
Clinical Presentation
LD presents a wide variety of symptoms, being erythema migrans (66.7%), fever (44.4%), extremities pain (38.9%), facial palsy (11.2%), paresthesias (11.2%), and joint pain (11.1%) (9) among the most frequent. Due to these non-specific symptoms, physical examination of patients does not reveal precise data. Symptoms appear before diagnosis in 66% of cases, with an average duration of 19.6 ± 9.3 (SD) days. Even though most clinical manifestations are not severe, 77.2% of cases required hospitalization, which highlights the need for a correct diagnosis and treatment (9). In short, the most common clinical manifestations are neurological, cutaneous, musculoskeletal and cardiac (4). These manifestations present a temporal progression through several LD stages. In the first stage (early localized LD), the most frequent manifestation is cutaneous, being erythema migrans a pathognomonic manifestation of the disease (12). In the pediatric population this symptom predominates in the upper half of the body, whereas in adults it is more common in the lower half (4). In the second stage (early disseminated LD), neurological symptoms and carditis appear. Furthermore, meningitis is a frequent complication caused by Borrelia infection, presenting a predominance of mononuclear cells and an increased cerebrospinal fluid (CSF) protein concentration. The third stage (late disseminated LD) is characterized by the development of chronic rheumatic conditions (4).It should also be noted that there is a high percentage of pediatric patients diagnosed with LD that do not develop a fever. This distinguishes borrelioses from other tick-borne diseases, which usually present recurrent fever.
With regard to neuroborreliosis, i.e., neurological involvement in LD, lymphocytic meningitis is the most usual manifestation, along with an increase in CSF proteins and lymphomonocytic pleocytosis. Radiculoneuritis and cranial neuropathy can also be present as early manifestations of the disease (13).
Treatment
Antibiotic therapy is the treatment choice for LD and there is a high response rate to it (4). Oral antibiotics are used in the early stages of the disease. Doxycycline is mostly employed in adults and amoxicillin in children under 8 and pregnant women. Moreover, ceftriaxone, cefuroxime, and amoxicillin-clavulanic acid have also been used with variable response rates (9). Intravenous treatment is reserved for severe cases of central nervous system involvement. Due to a possible chronification of symptoms, antibiotic therapy is long-lasting, although there is controversy regarding its duration (13).
NEED FOR DIAGNOSIS CONFIRMATION
Serological confirmation is necessary in order to establish a diagnosis of borreliosis, except in patients with erythema migrans, a pathognomonic sign of LD. In cases of clinical suspicion, the potential infection is to be confirmed through validated microbiological assays such as: IFA, ELISA (total antibodies, IgG and IgM), CLIA, PCR or culture (14). If positive or borderline antibodies are detected by IFA, ELISA or CLIA, these are to be confirmed by a Western blot (WB) assay (14). Due to the crossed reactions in ELISA or IFA serodiagnosis, a confirmation by WB (antibodies against IgG or IgM) is necessary. In addition, WB helps to know the specific antigens the antibody synthesis is directed towards.
Both a positive WB and the interpretation of the bands used on it (which depend on the analyzed antigens) are of great importance. This would help to know whether it is an active, persistent or past infection, and whether there are any infectious syndromes or cross-reactivity with other microorganisms.
PCR and culture of skin samples (skin biopsy) are extremely cost-effective in Reference Centers (14). This cost-effectiveness applies to the early stages of the disease, when patients still have negative auto-antibodies.
Blood samples are usually required for the above-mentioned assays, whereas CSF is required in cases involving signs of meningeal irritation or confirmed meningitis. Once the samples are collected, they are sent to the Reference Centers for analysis (14).
As previously explained in the section about the etiological variability of vectors and pathogens, ticks also carry other bacteria, especially Borrelia miyamotoi. To confirm a laboratory diagnosis, the presence of Borrelia miyamotoi must also be considered. This mainly applies to cases with a high level of suspicion for diagnosing LD, but testing negative for B. burgdorferi (10). Despite not being examined in conventional assays, spirochetes can be observed by darkfield microscopy (10).
DISCUSSION AND CONCLUSIONS
Some LD cases caused by other pathogens different from B. burgdorferi such as B. garinii are currently being reported in Spain. Nevertheless, there are not any confirmed cases of B. Miyamotoi up until now, since this species is not included into the conventional serologies. As a consequence, a new diagnostic protocol for LD is required, including new pathogen species to avoid delay in the diagnosis as well as the higher complication rate this inevitably implies.
In the differential diagnosis of a clinical picture with no obvious manifestations, LD should be considered as a possibility in patients with skin manifestations caused by confirmed or suspected tick-bite. This applies to cases involving no febrile clinical picture or a febrile clinical picture with no obvious origin, and/or to cases involving neurological syndrome without further explanation. A borreliosis screening is also required in cases involving musculoskeletal or cardiac manifestations with no obvious diagnosis, since patients may be in the late disseminated LD stage.
Large mammals are the main carriers of the genus Ixodes and, consequently, of potential borrelioses. Thus, a correct primary prevention by performing tests of livestock and household pets in the veterinary field is fundamental. Reducing the number of susceptible hosts (by means of a proper parasite treatment) will result in reducing the number of tick colonies. In addition, creating campaigns for the external parasite treatment of these animals would help to decrease the risk of livestock work, which is one of the main risk factors.
Primary prevention campaigns are also necessary in rural endemic areas, especially in coppice areas where climatic conditions are favorable for tick-bites. The campaigns should include recommendations to avoid human-tick contact, in particular for tourists and locals who are not used to the countryside. These recommendations should focus on wearing appropriate clothes such as trousers and closed shoes, so that the legs and feet are not exposed to the tick-bites. Furthermore, it is necessary to raise awareness on the importance of seeing a doctor after a tick-bite. The geographical area and the environment in which the patient lives, as well as the season of the year, can also be considered risk factors for a tick-bite.
Both a correct veterinary epidemiological surveillance (mainly focused on the livestock field) and an adequate description of the endemic areas facilitate the access to these data for medical personnel, facilitating a correct diagnosis. I A notification system of LD cases in which confirmed and suspected cases can be exhaustively registered would also be beneficial. Thus, the prevalence of LD in Spain could be determined more precisely.
STATEMENTS:
Acknowledgements
This paper is part of the Teaching Innovation Project coordinated between the Faculty of Medicine and the Faculty of Translation and Interpreting of the University of Granada (UGR), within the framework of the FIDO Plan 2018-2020 of the UGR (code 563).
Ethical concerns
This paper did not require the approval of any ethics committee.
Conflicts of interest
The authors of this paper declare no conflicts of interest.
Funding
No funding was received for the production of this paper.
REFERENCES
- Enfermedad de Lyme: MedlinePlus en español [Internet]. [Last access: 2 March 2020]. Available at: https://medlineplus.gov/spanish/lymedisease.html
- Taxonomy browser (Borrelia) [Internet]. [Last access: 2 March 2020]. Available at: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=138
- Bonet Alavés E, Guerrero Espejo A, Cuenca Torres M, Gimeno Vilarrasa F. Incidencia de la enfermedad de Lyme en España. Medicina Clinica. 2016 15;147(2):88–9.
- Vázquez-López ME, Pérez-Pacín R, Díez-Morrondo C, Díaz P, Castro-Gago M. Lyme disease in paediatrics. Anales de Pediatría. 2016 1;84(4):234–5.
- Portillo A, Santibáñez S, Oteo JA. Enfermedad de Lyme. Enferm Infecc Microbiol Clin [Internet]. 2014 [Last access: 2 March 2020];32(Supl 1):37–42. Available at: http://zl.elsevier.es
- Estrada-Peña A. CLASE ARACHNIDA Orden Ixodida: Las garrapatas. Revista IDE@-SEA, no [Internet]. [Last access: 2 March 2020];13:1–15. Available at: www.sea-entomologia.org/IDE@
- Estrada-Peña A, de la Fuente J. Host Distribution Does Not Limit the Range of the Tick Ixodes ricinus but Impacts the Circulation of Transmitted Pathogens. Frontiers in Cellular and Infection Microbiology [Internet]. 2017 Oct 11 [Last access: 2 March 2020];7(OCT):405. Available at: http://journal.frontiersin.org/article/10.3389/fcimb.2017.00405/full
- Blanco-Vidal MJ, Guio-Carrión L, Montejo-Baranda JM, Iraurgu-Arcarazo P. Neuroborreliosis: experiencia de 10 años en un hospital terciario del norte de España. Revista Española de Quimioterapia [Internet]. 2017 [Last access: 22 March 2020];30(3):234–5. Available at: https://medes.com/publication/122555
- Espí A, del Cerro A, Somoano A, García V, M. Prieto J, Barandika JF, et al. Borrelia burgdorferi sensu lato prevalence and diversity in ticks and small mammals in a Lyme borreliosis endemic Nature Reserve in North-Western Spain. Incidence in surrounding human populations. Enfermedades Infecciosas y Microbiologia Clinica. 2017 1;35(9):563–8.
- Palomar AM, Portillo A, Santibáñez P, Santibáñez S, Oteo JA. Borrelia miyamotoi: Should this pathogen be considered for the diagnosis of tick-borne infectious diseases in Spain? Enfermedades Infecciosas y Microbiologia Clinica [Internet]. 2018 1 [Last access: 22 March 2020];36(9):568–71. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29187292
- Vázquez-López ME, Pego-Reigosa R, Díez-Morrondo C, Castro-Gago M, Díaz P, Fernández G, et al. Epidemiología de la enfermedad de Lyme en un área sanitaria del noroeste de España. Gaceta Sanitaria. 2015 1;29(3):213–6.
- Comunicación Atención a pacientes con problemas infecciosos | Medicina de Familia. SEMERGEN | Medicina de Familia. SEMERGEN [Internet]. [Last access: 2 March 2020]. Available at: https://www.elsevier.es/es-revista-medicina-familia-semergen-40-congresos-39-congreso-nacional-semergen-55-sesion-atencion-pacientes-con-problemas-infecciosos-3726-comunicacion-eritema-migrans-44414
- Enfermedad de Lyme – Diagnóstico y tratamiento – Mayo Clinic [Internet]. [Last access: 2 March 2020]. Available at: https://www.mayoclinic.org/es-es/diseases-conditions/lyme-disease/diagnosis-treatment/drc-20374655
- Decálogo SEIMC de recomendaciones sobre el diagnóstico y tratamiento de la infección por Borrelia burgdorferi-E. de Lyme. Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica 2019.
- del Carmen Maroto Vela M, Gutiérrez Fernández J. Diagnóstico de laboratorio de la infección por Borrelia burgdorferi. Control de calidad SEIMC. Available at: http://www.aefa.es/wp-content/uploads/2014/04/Diagnostico-de-laboratorio-de-infeccion-por-borrelia.pdf
AMU 2020. Volumen 2, Número 1
Fecha de envío:
16/03/2020
Fecha de aceptación:
06/04/2020
Fecha de publicación:
29/05/2020