Cristina Morato-Gabao ¹ , David Navares-López ² , Rosa Pérez-Martínez ² , Laura Romero-de-los-Reyes ² , Lucía Solier- López ²
1 Estudiante del Programa de Doctorado en Psicología de la Universidad de Granada (UGR)
1 Student of the PhD Programme in Psychology at the University of Granada (UGR)
2 Student of the MSc in Basic Applied Neurosciences and Pain at the University of Granada (UGR)
TRANSLATED BY:
Marina Fernández-Basallo ³ , Alicia Gómez-Patiño ³ , Julia González-Cuenca ³ , María Repiso-Núñez ³ , María Pineda- Cantos ³ , Javier Saldaña-Martínez3
3 Student of the BA in Translation and Interpreting at the University of Granada (UGR)
In the last few years, localized neuropathic pain has reached a prevalence rate of over 50% in patients who have attended pain clinics. Moreover, 20% of the chronic pain that patients suffer is neuropathic. Therefore, it is necessary to provide an adequate definition, diagnosis, and intervention in order to deal with this syndrome and improve patients’ quality of life. The aim of this article is to carry out a review of the existing literature on this syndrome that has been published in recent years. Moreover, a complementary objective of this article is to create an approach to localized neuropathic pain by focusing on its pathophysiology, diagnosis, and treatment, which can be pharmacological (e.g. lidocaine and capsaicin in topical formulation) or non-pharmacological. In the end, there is a final section with a discussion and some future perspectives about the study of localized neuropathic pain.
Keywords: localized neuropathic pain, chronic pain, capsaicin, lidocaine.
- Introduction
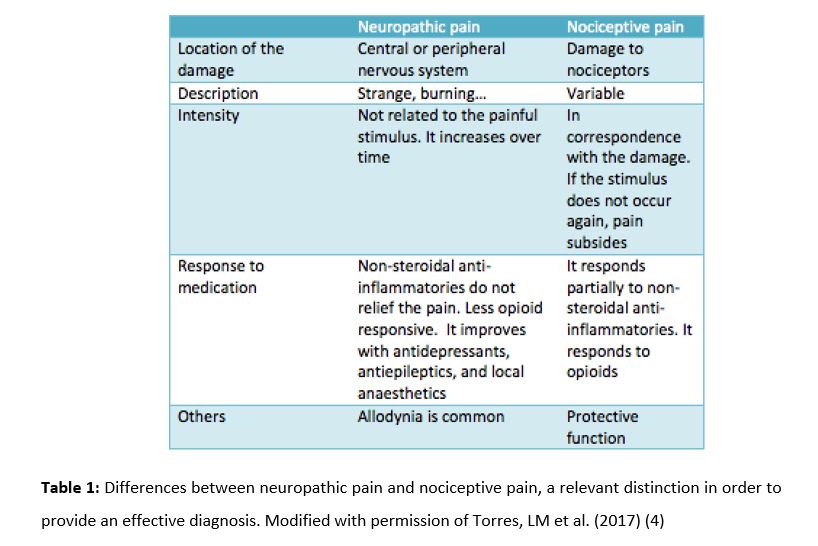
According to the International Association for the Study of Pain (IASP), pain is ”an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (1). Its purpose is to warn the organism. Meanwhile, neuropathic pain (NP) is a specific type of pain (syndrome) which is defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” (2). It can have a central origin (caused by spinal cord or brain damage) or a peripheral origin (caused by peripheral nerve damage), and it can be localized (if it affects a specific area of the body) or diffuse. It is essential to have a clear definition of every different pain syndrome to improve the classification of the patients, so that treatments can be adapted as specifically as possible (3). Table 1 shows the differences between NP and nociceptive pain.
A group of pain experts met in 2010 to create the first definition of localized neuropathic pain (LNP). They defined it as “a type of neuropathic pain that is characterized by consistent and circumscribed area(s) of maximum pain” (5). However, a more comprehensive definition is required to help to adapt better existing treatments to each patient, depending on the type of pain that they suffer and its location (3).
NP incidence was estimated between 6.9%-10% of the population (6). However, there is a lack of studies specifying the prevalence of each type of NP. It is important to highlight that 20% of chronic pain is neuropathic (7). Therefore, it is essential to diagnose and treat it properly in order to improve patients’ quality of life. According to the Guide for Diagnostic and Therapeutic Pharmacological Approach of Localized Peripheral Neuropathic Pain (4), a study carried out with patients treated in pain clinics showed an incidence of 51.9%. The majority were women and had a greater prevalence of peripheral NP, considerably higher than in other European countries. As reported by a survey conducted among doctors, the prevalence of LNP in their patients suffering NP was 60% on a sample of 869 people (5).
Several studies agree on the primary use of 5% lidocaine (8, 9), capsaicin (10, 11), clonidine, and botulinum toxin type A (BTX-A) for topical treatment (12, 13).
The purpose of this article is to carry out a review of the existing literature on LNP and to explore the pathophysiology, diagnosis, and treatment of this syndrome.
- Pathophysiology
NP is caused by a lesion or a disease which affects the somatosensory system (12). Such lesion or inflammation of peripheral tissues induces reversible adaptive changes in the nervous system that produce pain due to sensitization. This process acts as a protective mechanism to ensure the adequate healing of the tissues. In NP, changes in sensitization are persistent, causing spontaneous pain with a low stimulus threshold and even an onset or an increase of the pain with non-painful stimuli. This produces maladaptive changes on sensory neurons that can be irreversible (7, 14).
Some of the physiological changes that are produced in the peripheral area are electrical hyperexcitability and abnormal impulses (ectopic electrogenesis) (15) generated in the axon and in the injured primary sensory neurons in the dorsal root ganglion (7). Ectopia leads to spontaneous firing in some neurons and abnormal responses to mechanical, thermal, and chemical stimuli in many other neurons. The remodeling of voltage-sensitive ion channels, transducer molecules, and receptors in the cell membrane seems to be the cellular mechanism that underlies ectopic hyperexcitability (15). Na+ and K+ specific channels seem to have the greatest responsibility because they are directly involved in the repeated neuronal firing. Na+ channels accumulate in the membrane of injured nerve areas and in demyelination areas, the synthesis of the subtypes is increased, and its individual contribution could be increased by the mediators of hyperalgesia. In addition, this leads to a downregulation and to the block of K+ channels. The ectopic discharge contributes to NP as it generates a direct afferent signal and it can trigger and maintain central sensitization (15). Moreover, this causes the sensitization of nociceptors, the presence of ephapses, and abnormal interaction among fibers. At a central level, neurons of the posterior horn are sensitized and descending pain inhibitory mechanisms are altered (7).
LNP is also characterized by peripheral hyperexcitability, with overexpression of sodium and TRPV family 1 channels, which are located on nerve cell membranes. The analgesic effect of topical drugs used for NP treatment is particularly related to such channels, which are widely distributed on the surface of superficial or epidermal nociceptive fibers (16).
NP syndrome occurs as a complex combination of symptoms with interindividual variance that depends on the underlying pathophysiological changes resulting from the convergence of multiple etiological, genotypic, and environmental factors (7).
2.1 Medical signs of LNP
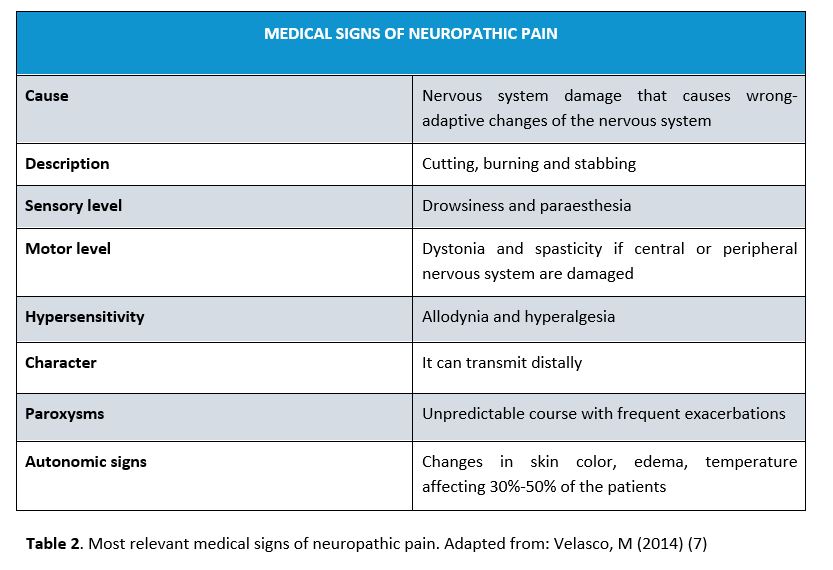
NP can present negative signs (e.g. sensory loss) or positive signs (e.g. abnormal neurosensorial responses). Somatization and sleep problems are also common, as well as mental disorders such as anxiety and depression. Negative signs are the first ones in determining that the somatosensory system is damaged. Positive signs can be either spontaneous (e.g. spontaneous pain, dysesthesia, paraesthesia) or evoked (e.g. allodynia, hyperalgesia, hyperpathia) (7). Table 2 shows the most relevant medical signs.
- Diagnosis
NP and LNP follow the same diagnosis. According to authors such as Finnerup et al. (17) and the IASP (1), if a patient suffers from a pain that could be the result of a neurologic lesion or disease instead of a lesion in the tissue, it must be classified as a possible, probable or definite pain.
3.1. Possible neuropathic pain
Firstly, the patient’s health record must be checked searching for a neurologic disease or lesion. The health record must show the pain history, suffered diseases, and existing comorbidities. In order to determine the pain level suffered by the patient, a visual analogue scale or a numerical scale can be used (18), as well as other scales or questionnaires (17, 19). Electrophysiological techniques such as electromyography (EMG), nerve conduction study or small fibre tests can be carried out (7). A physical examination is advisable to locate the painful area (18). In order for a patient to be diagnosed with possible LNP, two criteria must be met: (1) the potential existence of a serious neurologic problem (e.g. ictus, diabetic neuropathy) must be checked, and (2) the anatomical location of the pain must be determined in order to decide if it is compatible with the location of the lesion in the central or peripheral somatosensory nervous system (17). Pain can appear immediately or insidiously, depending on the lesion or disease, and the time relationship is relevant for the diagnosis (17).
3.2. Probable neuropathic pain
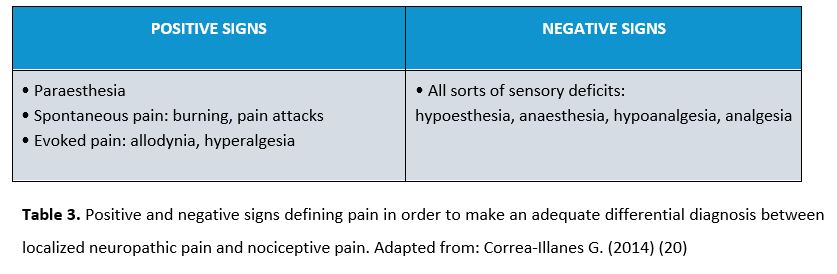
In this case, enough clinical support is needed to confirm what has been previously found. Despite the existence of positive signs, negative signs will determine LNP diagnosis (17). Table 3 shows positive and negative signs of NP.
In order for a patient to be diagnosed with probable LNP, the following scales and questionnaires must be positive: the LANSS scale, the Neuropathic Pain Questionnaire, the DN4 questionnaire, painDETECT or ID-Pain (17). Currently, there is a new specific tool for LNP called Diagnostic Tool, which enables to determine the localized character of NP (4) and which must also be positive to ascertain that NP is probable.
3.3. Definite neuropathic pain
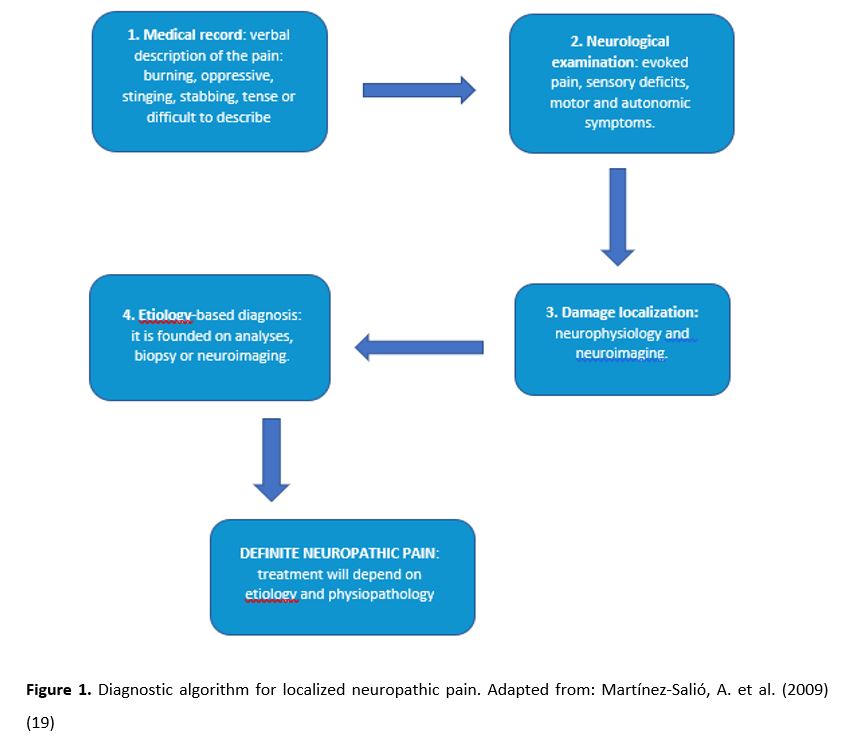
The diagnosis must be based on medical imaging techniques which describe the lesion in the somatosensory nervous system, including: magnetic resonance imaging (MRI), computed tomography (CT) scan (17), neurophysiological tests (e.g. EMG, nerve conduction) (4), evoked potentials, quantitative sensory testing (QST), and withdrawal reflexes, among others. It should be noted that there can be other pain causes, and experts may not know if there is a direct causality (17). Figure 1 shows a diagnostic algorithm facing NP.
- Treatment
4.1. Pharmacological treatment
Pharmacological treatment for chronic illnesses has the disadvantage of a long-term low compliance by patients with the treatment. In addition, the effectiveness of pharmacological treatment for NP is limited, and only 40% of the patients experience a significant relief (16). Topical targeted treatment is administered for LNP, mainly 5% lidocaine and 8% capsaicin (3).
4.1.1. 5% lidocaine
5% lidocaine patches are a first-line treatment. They present a pharmacological action through lidocaine and a protective action by means of the patch, which acts as a physical barrier before the stimuli causing hyperalgesia (21). Lidocaine carries out a non-selective blockade of Na+ channels, and it attaches to the pores of the small local fibres damaged. This blockade halts signal propagation. Nevertheless, the final action will depend on the affinity and the binding rate of the drug, since there is not a complete sensory blockade of Na+ channels (21). Lidocaine patches reduce allodynia and NP symptoms (3).
The half-life of lidocaine is 7.6 hours. Therefore, it must be administered every 24 hours to keep its analgesic effect (16). The most common adverse effects of lidocaine are erythema, burning sensation, rashes, edema, and dermatitis, and they are limited to the application area (16).
4.1.2. 8% capsaicin
When neuropathy is caused by post-herpetic neuralgia (PHN) or the human immunodeficiency virus (HIV), capsaicin patches have been given the level A of efficiency by the European Federation of Neurological Sciences (16). Capsaicin interacts with the afferent nerve fibres through the selective agonist affinity for TRPV1, mainly located in Aδ-fibres, C-fibres and intracellular organelles (16). Capsaicin action is mediated by the opening of the TRPV1 channel and the subsequent depolarization through Na+ and Ca2+, as well as Ca2+ liberation to the endoplasmic reticulum (22). The high concentration of Ca2+ blocks the afferent nerves selectively. Nociceptive pain symptoms improvement occurs between 6 and 12 weeks by using a single 8% capsaicin patch (23). The drug effects last up to 90 days, so it is administered every three months (16).
4.1.3. Other topical and non-topical targeted treatments
Several topical targeted treatments for LNP are not yet commercially available. One of them is ketamine, an N-Methyl-D-aspartic acid (NMDA) receptor agonist that reduces the threshold of nerve transduction and central sensitization (16). Ketamine is not approved for LNP treatment, although its effectiveness has been demonstrated (3).
Dextromethorphan is a non-competitive NMDA receptor antagonist, marketed as an external patch whose function is to relieve both muscular and rheumatic pain (16). Another example is bupivacaine, which is a local anaesthetic that blocks Na+ channels, also marketed as a long acting patch that provides an anaesthetic effect for a period of up to 3 days after a single application. Its effect is compared to 5% lidocaine patches (24). Furthermore, diclofenac and ketoprofen are nonsteroidal anti-inflammatory drugs (NSAIDs) available as patches and creams to treat chronic pain (24). In addition, the use of μ fentanyl agonist opioids and the partial µ-agonist buprenorphine has not been tested in LNP, although its effectiveness has been demonstrated in chronic cancer cases and non-cancer pain (3).
On the other hand, oral antidepressants and antiepileptics are also recommended for LNP treatment. Although there are numerous antidepressant and antiepileptic drugs, rotigotine and amitriptyline are the only ones that have been evaluated to treat pain conditions. The most commonly used antiepileptic drugs in LNP treatment are gabapentin and pregabalin, both administered orally (24).
In conclusion, the 5% lidocaine patch and the 8% capsaicin patch are the only topical dressings that are specific for treating LNP nowadays (16).
4.2 Non-pharmacological treatment
As of today, there is little evidence of the use of non-pharmacological treatment for LNP. However, the few existing data recommend its use as an attempt to reduce the use of medications and improve patients’ quality of life (25).
4.2.1 Interventional therapies
Spinal cord stimulation is a low-intensity electrical stimulation of large myelinated Aβ-fibers that modulates the pain signals from non-myelinated C-fibers (26). It is not only the most commonly used neuromodulation strategy, but also the most researched. It is based on the application of a monophasic square-wave pulse, which causes paresthesia in the affected region. New types of stimulation, such as burst and high frequency stimulation, can produce a stimulation without paresthesia and an equal pain relief compared with the monophasic square-wave pulse. These techniques are relatively safe, and their long-term effectiveness has been demonstrated by randomized controlled trials and several cases (26).
Dorsal root ganglion intervention, peripheral nerve intervention, and peripheral nerve field stimulation are three therapies based on the neurostimulation of afferent fibres outside the spinal cord and the subcutaneous stimulation of the peripheral nerve area. A cohort research reported that stimulation provided about 50% of pain reduction. (26)
Epidural motor cortex stimulation (EMCS), repetitive transcranial magnetic stimulation (rTMS), and transcranial direct current stimulation (tDCS) of central motor cortex at levels below the motor threshold (27) can reduce thalamus hyperactivity or activate descending inhibitory pathways. Data appear to indicate that 60% of patients respond to EMCS (28).
Moreover, the internal capsule, nuclei in the sensory thalamus, the periaqueductal gray substance, the motor cortex, the septum, the accumbens, the posterior hypothalamus, and the anterior cingulate cortex have been suggested as potential target areas in deep brain stimulation. However, the application of this technique is controversial due to the significant risks showed by the current evidence (26).
4.2.2 Physical therapies
Although there is little evidence of the effectiveness of physical therapy, there are signs indicating that physical exercise and movement representation techniques (i.e. treatments that use the observation or imagination of normal pain-free movements, like mirror therapy and motor imagery) are beneficial (29).
4.2.3 Psychological therapies
The main goal of these therapies is to promote pain management and to reduce its emotional consequences. Cognitive-behavioral therapy (CBT), whose purpose is to lead the patient to an “individual change”, is the most researched therapy. This treatment addresses mood, function (including disability) social engagement, and analgesia as an indirect target. There are not enough evidences of the effectiveness of these therapies in LNP treatment, so an in-depth research of this field is required (30).
- Discussion and future perspectives
Nowadays, we face multiple challenges in relation to LNP. It is very hard to make a correct diagnosis of LNP due to the lack of consensus regarding its definition. Although there are several debates going on between experts (5, 16), there is not a global consensus on the definition of LNP, making an adequate diagnosis difficult. Another problem that needs to be solved is knowing the specific treatments that could be useful against the different types of NP (3), since there are patients with a treatment that does not effectively adjust to their pathology. Further research on the pathophysiological characteristics of LNP could help in the development of new effective and specific treatments for this type of pain.
In conclusion, an in-depth study of LNP is required in order to increase our knowledge about it, focusing on three aspects: (1) a global consensus on its definition, (2) more research on its physiology, and (3) more research on the effective treatments for each type of NP.
These aspects could enhance the adequacy of treatments, improving their effectiveness and patients’ quality of life, given the chronicity of this condition.
Conflicts of interest statement
The authors declare that there are no conflicts of interest in this article.
References
- Merskey H, Bogduk N. Classification of Chronic Pain, Second Edition, IASP Press, Seattle, 1994. http://www.iasp-pain.org.
- Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008; 70(18): 1630-1635. Doi: 10.1212/01.wnl.0000282763.29778.59
- Rey R. Tratamiento del dolor neuropático. Revisión de las últimas guías y recomendaciones. Neurolarg. 2013; 5(S1): S1–S7. Doi: 10.1016/j.neuarg.2011.11.004
- Torres LM, Galvez R, Calderon E. Guía para el abordaje diagnóstico y terapéutico farmacológico del Dolor Neuropático Periférico Localizado (DNL). España: Asociación Andaluza del Dolor; 2017.
- Mick G, Baron R, Finnerup NB, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manag. 2012; 2(1): 71-77. Doi: 10.2217/pmt.11.77
- Cruccu G, Truini A. A review of Neuropathic Pain: From Guidelines to Clinical Practice. Pain Ther. 2017; 6(1): 35-42. Doi: 1. 10.1007/s40122-017-0087-0
- Velasco M. Dolor neuropático. Med. Clin. Condes. 2014; 25(4): 625-634. Doi: 10.1016/S0716-8640(14)70083-5
- Amato F, Duse G, Consoletti L, et al. Efficacy and safety of 5% lidocaine-medicated plasters in localized pain with neuropathic and/or inflammatory characteristics: An observational, real-world study. Eur Rev Med Pharmacol Sci. 2017; 21(18): 4228-4235. https://www.europeanreview.org/wp/wp-content/uploads/4228-4235-5-lidocaine-medicated-plasters-in-localized-pain.pdf
- Baron R, Allegri M, Correa-Illanes G, et al. The 5% lidocaine-medicated plaster: its inclusion in international treatment guidelines for treating localized neuropathic pain, and clinical evidence supporting its use. Pain The 2016; 5(2): 149-169. Doi: 10.1007/s40122-016-0060-3
- Bhaskar A, Mittal R. Local therapies for localised neuropathic Pain. Rev pain. 2011; 5(2): 12-20. Doi:1177/204946371100500203
- Üçeyler N, Sommer C. High-dose capsaicin for the treatment of neuropathic pain: what we know and what we need to know. Pain Ther. 2014; 3(2): 73-84. Doi:1007/s40122-014-0027-1
- Allegri M, Baron R, Hans G, et al. A pharmacological treatment algorithm for localized neuropathic pain. Curr. Med. Res. Opin. 2016; 32(2): 377-384. Doi: 10.1016/j.semerg.2016.10.004
- Zur E. Topical treatment of neuropathic pain using compounded medications. Clin J Pain. 2014; 30(1): 73-91. Doi: 10.1097/AJP.0b013e318285d1ba
- Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2017; 18: 20-30. Doi: 1038/nrn.2016.162
- Devor M. Respuesta de los nervios a la lesión en relación con el dolor neuropático. En: McMahon SB, Koltzenburg M, editores. Wall y Melzack Tratado del dolor. 5ª Ed. Madrid: Elsevier; 2007. 927-951
- Pickering G, Martin E, Tiberghien FL, Delorme CL, Mick G. Localized pain: an expert consensus on local treatments. Drug Des Devel Ther. 2017; 11: 2709–2718. Doi: 10.2147/DDDT.S142630
- Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016; 157(8): 1599-606. Doi: 10.1097/j.pain.0000000000000492
- Fernández R, Ahumada, M et al. Guía para definición y manejo del dolor neuropático localizado: Consenso Chileno. El Dolor. 2011; 55: 12-31. https://www.ached.cl/upfiles/revistas/documentos/4fe37b78dcb16_dnl_55.pdf
- Martínez-Salió A, Gómez A, Ribera MV, et al. Diagnóstico y tratamiento del dolor neuropático. Med Clin. 2009; 133(16): 629–636. Doi:10.1016/j.medcli.2009.05.029
- Correa-Illanes G. Dolor Neuropático, clasificación y estrategias de manejo para médicos generales. Revista clínica médica las condes. 2014; 25(2): 189-199
- Sheets MF, Hanck DA. Molecular action of lidocaine on the voltage sensors of sodium channels. J Gen Physiol. 2003; 121(2): 163–175. Doi: 1085/jgp.20028651
- Derry S, Lloyd R, Moore RA, McQuay HJ. Topical capsaicin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2009; (4): CD007393. Doi: 10.1002/14651858.CD007393.pub2
- Sawynok J. Topical analgesics for neuropathic pain: preclinical exploration, clinical validation, future development. Eur J Pain. 2014; 18(4): 465–481. Doi: 10.1002/j.1532-2149.2013.00400.x
- Lodge D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009; 56(1): 6–21. Doi: 10.1016/j.neuropharm.2008.08.006
- Zilliox LA. Neuropathic pain. Continuum Minneap Minn. 2017; 23(2): 512–532. Doi: 10.1212/CON.0000000000000462
- Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat. Rev. Dis. Primers. 2017; 3: 1-19. Doi: 10.1038/nrdp.2017.2
- Sukul VV., Slavin KV. Deep brain and motor cortex stimulation. Curr Pain Headache Rep. 2014; 18(7): 1-5. Doi: 10.1007/s11916-014-0427-2
- Lefaucheur JP. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain. 2016; 157: S81–S89. Doi: 10.1097/j.pain.0000000000000401
- Eccleston C, Fisher E, Craig L, Duggan GB, Rosser BA, Keogh E. Psychological therapies (internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014; 2: CD010152. Doi: 10.1002/14651858.CD010152.pub2
- Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014; 8: 1-9. Doi:3389/fncel.2014.00102