Ramos Cela, Miguel¹; Medina Martínez, Alberto Jesús¹; Vera Martín, Ignacio¹ *
1 Universidad de Granada, Facultad de Medicina
* Corresponding Author: i.veram@alumnos.upm.es
Abstract
Listeria monocytogenes (LM) is a common cause of Central Nervous System (CNS) infections, especially in immunosuppressed patients, infants, and elderly people. The fact that makes LM a dangerous bacterium is that it can easily evade the immune system and be transmitted by the fecal-oral route causing a malignancy called listeriosis. Nonetheless, the current incidence of a Listeria monocytogenes infection is 3-6 cases per million, but its prevalence has been increasing. These bacteria become especially pathogenic when infecting the CNS, the reason why neurolisteriosis can account for as much as half the cases of invasive listeriosis, the remaining cases being usually isolated bacteremia or disease in pregnancy.
There is an ample list of risk factors related with listeriosis such us malignancies, alcoholism and/or liver disease, HIV/AIDS, or diabetes, but only some of them are closely interconnected with neurolisteriosis. These are the hormonal environment in pregnancy, aging, corticotherapy and immunosuppressive comorbidities. In this review we focus on the LM infection pathways and the main risk factors that let this bacteria act and generate nervous system-associated complications.
Keyword: Listeria monocytogenes, immune system, listeriosis, neurolisteriosis, Central Nervous System, risk factors.
1. Introduction
Listeria monocytogenes (LM) is a foodborne Gram + bacillus which causes listeriosis, having the highest hospitalization rate among foodborne diseases (1). It is the only known Listeria species with pathogenic potential. Human listeriosis manifests as septicemia, CNS invasion, which is called Neurolisteriosis (NL), and maternal-fetal infections, as well as rare forms of localized infections. CNS disorders associated with Listeriosis are especially concerning as they are estimated to represent about one third of the total cases (2).
LM can be found in a wide range of environments such as soil, water and feces. It can colonize plants, birds and an ample group of mammals. This large presence in nature favors the infection of livestock and crops, through which it can easily enter the human food chain supply and pose as a sanitary threat (3). Although its existence was discovered as early as the 1920s and after World War II it caused terrible outbreaks of fatal CNS infections and miscarriages, it was not until 1983 when its food-borne transmission was proven and fully understood (4). Since then, LM infections have escalated quickly as a major human epidemiologic issue due to the vast industrialization of the alimentary business and rapid distribution of its products, the general habit of refrigerating food (which enables LM growth), and the increased number and life-span of immunosuppressed patients (due to the increased number of newly approved immunosuppressive drugs), who are the principal risk population for listeriosis (5). This illustrates the need of implementation of well-standardized, microbiological and epidemiologic surveillance programs, which are extremely effective to prevent large-scale outbreaks. In fact, it has been in poorly developed countries, which lack the mentioned strategies, where the most destructive outbreaks have taken place, such as the one in South Africa in 2017 which is the largest ever known still to this day (6).
Neurolisteriosis: epidemiology and treatment
Bacteremia and Neurolisteriosis (NL) are the deadliest complication of listeriosis infection, being NL the one that causes more disability in perinatal and not perinatal patients such as intellectual disability, epilepsy, motor impairment, vision loss and stroke (7). The treatment of infection by LM is different depending on if neural affectation exists. The MONALISA study found that the adjunctive treatment with corticoids such as dexamethasone increment the mortality of patients with NL meanwhile the use of Cotrimoxazole, due to this penetration in the NS, is a favorable alternative in these patients (2). In a similar way, Linezolid could be other suitable option due to his high penetration in the Cerebrospinal Fluid (CSF) (8). Unsurprisingly, it has been demonstrated that a delay in the treatment of NL is strongly associated with worse outcomes (9).
Diagnosis and clinical presentation
LM infection is often misdiagnosed because the prodromal symptoms are nonspecific and meningeal signs are uncommon. In the case of neuroinvasion, LM can cause meningitis, meningoencephalitis, or abscess formation in the brain and spinal cord (10). Rhombencephalitis is a particular form of encephalitis listeriosis affection that affects primarily the brain stem and cerebellum (rhombencephalon). Neurolisteriosis prognosis is especially concerning: 30% of the individuals who develop this clinical manifestation have a 3-month mortality rate, and above 60% of patients never fully recover (2). Moreover, as we mention before, neurolisteriosis is almost always related to some other comorbidity, mostly associated with some type of immunosuppression (11).
Therefore, neurolisteriosis is the worst clinical outcome following a Listeria monocytogenes infection, as it entails high morbidity and mortality. Although some risk factors for contracting this infection are known, there is still no solid evidence on them. The aim of this paper is to review CNS infections caused by LM, with special emphasis on risk factors associated with a worse clinical course. It is of paramount importance to understand and evaluate these risk factors to establish better diagnostic methods in order to improve the future treatment the individual may receive, by broadening our global knowledge of the causes that ultimately lead to neural-related physiological impairments.
2. Physiological and molecular pathogenic features of Listeria
Clinical symptoms after infection can be quite severe and diverse due to the physiological characteristics of the colonization process: the bacterium is able to cross the intestinal barrier, the blood-brain barrier, and the placental barrier in pregnant women, repeating the process in the fetus (12). LM can also cause invasion of the CNS through a retrograde neural route (11). A striking feature of LM is that most of its life cycle occurs in the cytoplasm of the cells that it has tropism for. This is promoted by a bacterial invasion protein exposed in its surface which induces phagocytosis in cells that are normally non-phagocytic. Immunocompromised hosts, specifically with deficient Cell- mediated immunity (CMI), are the most prone to neurolisteriosis. In this scenario, LM can rapidly multiply unrestrictedly in the hepatocytes from which they further disseminate hematogeneously to the brain and elsewhere. The physiological path inside the organism, as well as the mechanism that the bacterium employs to enter the cell, escape from the phagocytic vacuole, and spread from one cell to another through actin-based motility are all summarized in Figure 1 and 2.
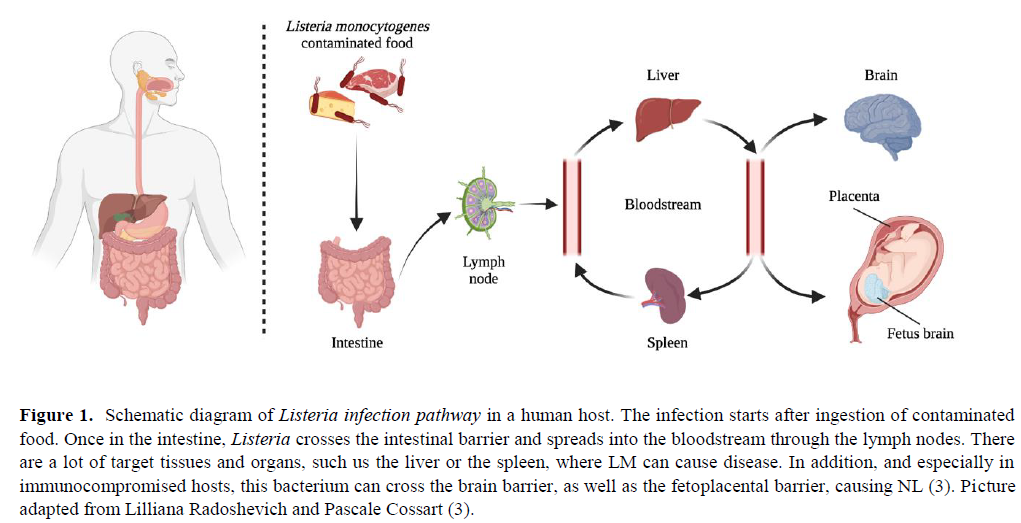

The non-phagocytic bacterial entry is mediated by at least two factors: Internalin A (InlA) and B (InlB). The first step occurs in non-phagocytic cells such us epithelial cells through receptor-mediated endocytosis and that is why this bacterium can spread through epithelial barriers. Exit from the vacuole requires expression of Listeriolysin O (LLO), a pore-forming toxin which in some cells can function synergistically with or be replaced by a phosphatidylinositol-specific phospholipase C (PI-PLC). The pore-forming activity of extracellular LLO leads to internal changes in cell processes distinctives of the Listeria infection. These include changes in histone modification, deSUMOylating, mitochondrial fission, endoplasmic reticulum (ER) stress and lysosomal permeabilization (13). The vacuole escape happens more often than the transcytosis process, and lysis of the two- membrane vacuole is performed by Lecithinase A (PlcA) and B (PlcB). On the other hand, in goblet cells, it can transcytose across the cell within a vacuole, and in some macrophages, it can replicate in spacious Listeria-containing phagosomes (SLAPs) (14). The majority of genes coding for these virulence factors are clustered in the PrfA regulon, located on 10 kb region of the bacterial chromosome. The PrfA regulon takes its name because all known virulence genes are under the either absolute or partial control of a pleiotropic activator protein PrfA (14). This cluster is represented in Figure 2 and the structure of the PrfA transcript is shown in Figure 3. Upon vacuolar escape, the intracellular movement of the bacteria requires expression of actin assembly-inducing protein (ActA) and polymerization of actin. This molecule enables the actin-based motility which is the way the aggressor can spread from cell to cell.

The activity of potent virulence factors causes a plethora of effects in the infected mammalian cell. Goblet cells are simple columnar epithelial cells that secrete gel-forming mucins, like mucin MUC5AC (15). In this cells Listeria nuclear targeted protein A (LntA) interacts with the Bromo adjacent homology domain-containing 1 protein (BAHD1) complex and induces deacetylation in histone 3 at lysine 18, leading to changes in chromatin packing. This modification is going to alter downstream gene expression. Furthermore, infection also leads to DNA damage. The host cell way to fight against Listeria infection is by upregulating several antibacterial effectors, for example, ISG15, which is the main actor of the ISGylation process that is based on a covalent modification of ER and Golgi proteins which modulate expression of cytokines IL6 and IL8 (16).
3. Relevant risk factors
Hormonal environment during the process of pregnancy creates a local suppression of CMI at the maternal foetal interface (17). Therefore, LM can reach the fetus from the maternal blood by the placenta. LM can also infect the baby during delivery or through a nosocomial transmission (18). The sign and symptoms of this infection include disseminated granulomatous lesions with micro abscesses (19) and hydrocephaly and delayed neurologic development (20). Although most cases are seen during second and third trimester, possibly, this reflects a bias where the early fetal losses caused by LM are not normally diagnosed (12). On the other hand, it is generally believed that the NS of the mother has not an increased risk of infection because of being pregnant. In fact, this has only been seen very exceptionally (21).
Elderliness has been demonstrated to be a strong risk factor to NL. This is caused by the partial loss of T-cell immunity that occurs in the process of aging. Despite this, bacteremia is more frequent since the age of 75 onwards, whereas NL appears more in the interval of 45-65 years old (22)
Systemic lupus erythematosus (SLE) is an autoimmune condition in which healthy cells and tissues are attacked throughout the organism, and infection is one of the main causes in these patients. A report of 26 cases in Mexico with SLE (23) found that, although among SLE patients neurolisteriosis was extremely rare (0,53-2,25%), its clinical presentation is unspecific, and its diagnosis can be easily mistaken with a neuropsiquiatric lupus outbreak. LM is actually one of the top three pathogens causing CNS infections in SLE patients (24). There is not enough evidence, however, to confirm if SLE increases the risk of LM infection, but it is clear that it worsens its prognosis. Studies (25)indicate that early diagnosis from blood and tissue cultures determine the effectiveness and success of the future treatment.
In a prospective study of 16 million people in the Netherlands (25) they found that of all bacterial meningitis among diabetic patients over 6% were caused by LM, and that these patients were at a 2-fold higher risk of developing meningitis, due to an impaired cell-mediated immunity with decreased efficacy of polymorphonuclear leukocytes, monocytes, and T-lymphocytes.
Other case report (26) finds the development of rhombencephalitis after a Listeria infection, in which the patient medical history included hypertension, type II diabetes and more importantly cryptogenic cirrhosis. As in almost every other case report, they outline the importance of an early diagnosis for future antibiotic treatment and an urgent MR brain scan for patients with progressive disabling brainstem signs.
Multiple Sclerosis (MS) by itself is not considered a risk factor for developing neurolisteriosis but its treatment includes alemtuzumab, an anti-CD52 monoclonal antibody that causes a massive CD8+ T-cell depletion, which may enhance LM replication after infection (27). So far, very few cases of MS patients under alemtuzumab treatment were reported to develop neurolisteriosis, but it is believed by the authors that these cases might be heavily underrepresented. For all this it is especially important to follow closely in MS patients the outbreak of LM infections, because most of them might be healthy carriers before alemtuzumab treatment, as they developed the disease in a matter of hours after administration. In these cases, the best option is believed to be an antibiotic prophylactic treatment.
The MONALISA study (2) found that patients with neurolisteriosis treated with adjuvant corticoids had a worse outcome in comparison with patient without corticotherapy. As explained before, LM is an intracellular facultative bacterium. Therefore, a suppression or decrease in the CMI is going to facilitate its dissemination and neuroinvasion. According to the MONALISA study, statistically the most frequent comorbidities which increase the chance of developing neurolisteriosis are solid organ cancer and diabetes mellitus.
In this study they also describe all the possible immunosuppressive causes for NL which are: daily alcohol intake (which stands for more than three drinks per day), cirrhosis, diabetes mellitus, end-stage renal disease, solid organ cancer, hematological neoplasias, hematopoietic stem- cell transplantation, solid organ transplantation, asplenia, pre- existing neutropenia, pre-existing lymphopenia, HIV infection, inflammatory bowel diseases, inflammatory rheumatic disorders, other autoimmune diseases and congenital immune deficiency (2).
4. Conclusions
This review is a study with certain limitations. When speaking about risk factors for bacterial infections, there are many that are common to different infections in different parts of the body. Since our work is focused on the risk factors of Neurolisteriosis, there are not many papers that gather such specific data, and they tend to focus either on more diverse symptomatology or even other infections caused by other bacteria.
There is a series of risk factors such as alcoholism, diabetes, bad nutritional habits, etc. that are general for listeriosis. Therefore, we decided not to analyse all of them and focus exclusively on the risk factors specific for Neurolisteriosis. It is important to clarify that in this sense, the literature review is neither complete nor perfect. It could be improved by doing a meta-analysis of all the risk factors related to listeriosis and not only those exclusive to CNS impairments.
However, the future of Neurolisteriosis research is promising as there is still much information to be extracted from a typical LM infection in the Central Nervous System. As a matter of fact, our work has evidenced an increased trend of investigation within this field of study, and as more clinical cases keep being published the better understanding of this disease the scientific community will have. Hence, this review could help future researchers to more easily organize the information gathered on Listeria monocytogenes and the risk factors associated with its neuronal infection.
Neuroinvasion due to L. monocytogenes can cause several forms of encephalitis and meningitis with diverse clinical manifestations, and as of today LM remains an important public health issue, particularly in the elderly, infants, immunosuppressed, and those with malignancies that may influence in any way the immune system function. For patients with a clinical diagnosis of rhombencephalitis and acute bacterial meningitis, recognition of the symptoms caused by listerial infection plays a vital role in allowing early diagnosis, treatment and ensures an optimal patient outcome without neurologic sequelae.
Since LM is only sensitive to certain antibiotics, it is important to perform an early microbiological diagnosis that confirms the infectino. LM is difficult to isolate from the CSF but not so much from blood or other infected tissues. In addition to the microbiological diagnosis, MR imaging is extremely important in demonstrating the predilection of the listerial infection for the brain stem and cerebellum.
As it has been previously explained, several scenarios exist where this infection can be considerably more dangerous and pose a life-threatening situation to the patient. In pregnant women diagnosis and detailed treatment tracing by doctors is essential, as it is at risk not only the life of the woman but also the foetus, with the additional complications a miscarriage or stillbirth may cause. Other than that, patients with impairedimmune system can exist due to a wide variety of factors. Immunosuppressive treatments such as corticosteroids or monoclonal antibodies can worsen the course of a pre- existing or novel infection, and in these cases an antibiotic prophylactic treatment is of urgent need following an early diagnosis. Autoimmune conditions such as Lupus Erythematosus are also among the list of risk factors that can influence the development of neuroinvasive situations by LM, with very unspecific symptomatology.
For all of the exposed above, among these patients an early diagnosis is even more relevant and can change the course of infection. Precise evaluation of symptomatology and continuous monitoring should be always performed. The CSF in listerial infection typically reveals an increased leukocyte count, usually with the predominance of polymorphonuclear cells, increased protein, and normal glucose levels. Moreover, although it is complicated, increased and improved cell culture methods should be developed to confirm LM presence, especially in CSF. And lastly, antibiotic prophylactic treatment should be considered when patients are suspects of being healthy carriers.
Statements
Acknowledgements
We gratefully acknowledge teachers Pablo Redruello Guerrero, Mario Rivera Izquierdo and Antonio Jesús Láinez Ramos-Bossini for their help to conduct the present studies.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
None
Abbreviations
- Acquired Immunodeficiency Syndrome (AIDS)
- Actin assembly-inducing protein (ActA)
- Bromo adjacent homology domain-containing 1 protein (BAHD1)
- Cell-mediated immunity (CMI)
- Central Nervous System (CNS)
- Cerebro Spinal Fluid (CSF)
- Human Immunodeficiency Virus (HIV)
- Interleukin 6 (IL6)
- Interleukin 8 (IL8)
- Internalin A (InlA)
- Internalin B (InlB)
- Lecithinase A (PlcA) and B (PlcB)
- Listeria nuclear targeted protein A (LntA)
- Listeria monocytogenes (LM)
- Listeriolysin O (LLO)
- Magnetic Resonance (MR)
- Mucin 5AC gene (MUC5AC)
- Multiple Sclerosis (MS)
- Neural System (NS)
- Neurolisteriosis (NL)
- Phosphatidylinositol-specific phospholipase C (PI-PLC)
- Transcription Factors (TFs)
References
1. Hedberg Foodborne Illness Acquired in the United States (Response). Emerging Infectious Diseases. 2011;17(7):1338–1338.
2. Charlier C, Perrodeau É, Leclercq A, Cazenave B, Pilmis B, Henry B, et Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. The Lancet Infectious Diseases. 2017;17(5):510–9.
3. Walland J, Lauper J, Frey J, Imhof R, Stephan R, Seuberlich T, et Listeria monocytogenes infection in ruminants: Is there a link to the environment, food and human health? A review. Schweiz Arch Tierheilkd. 2015;157(6):319–28.
4. Schlech New perspectives on the gastrointestinal mode of transmission in invasive Listeria monocytogenes infection. Clinical and investigative medicine Medecine clinique et experimentale. 1984;7(4):321–4.
5. Lecuit M. <scp> Listeria monocytogenes </scp> , a model in infection biology. Cellular Microbiology. 2020;22(4).
6. Thomas J, Govender N, McCarthy KM, Erasmus LK, Doyle TJ, Allam M, et al. Outbreak of Listeriosis in South Africa Associated with Processed Meat. New England Journal of 2020;382(7):632–43.
7. de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, et al. The global burden of listeriosis: a systematic review and meta- The Lancet Infectious Diseases. 2014; 14(11):1073–82.
8. Callapina M, Kretschmar M, Dietz A, Mosbach C, Hof H, Nichterlein Systemic and Intracerebral Infections of Mice with Listeria monocytogenes Successfully Treated with Linezolid. Journal of Chemotherapy. 2001;13(3):265–9.
9. Arslan F, Meynet E, Sunbul M, Sipahi OR, Kurtaran B, Kaya S, et al. The clinical features, diagnosis, treatment, and prognosis of neuroinvasive listeriosis: a multinational European Journal of Clinical Microbiology & Infectious Diseases. 2015;34(6):1213–21.
10. Drevets DA, Leenen PJM, Greenfield RA. Invasion of the central nervous system by intracellular Clin Microbiol Rev. 2004;17(2):323–47.
11. Clauss HE, Lorber Central nervous system infection with Listeria monocytogenes. Current Infectious Disease Reports. 2008 Jul 11;10(4):300–6.
12. Charlier C, Disson O, Lecuit M. Maternal-neonatal Virulence. 2020;11(1):391–7.
13. Cossart P, Lecuit Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. The EMBO Journal. 1998;17(14):3797–806.
14. Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and Nature Reviews Microbiology. 2018;16(1):32–46.
15. Ma J, Rubin BK, Voynow JA. Mucins, Mucus, and Goblet Chest. 2018;154(1):169–76.
16. Krypotou E, Scortti M, Grundström C, Oelker M, Luisi BF, Sauer-Eriksson AE, et Control of Bacterial Virulence through the Peptide Signature of the Habitat. Cell Reports. 2019;26(7):1815-1827.e5.
17. Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and Susceptibility to Infectious Diseases. Infectious Diseases in Obstetrics and 2013;2013:1–8.
18. MYLONAKIS E, PALIOU M, HOHMANN EL, CALDERWOOD SB, WING EJ. Listeriosis During Medicine. 2002;81(4):260–9.
19. Janakiraman V. Listeriosis in pregnancy: diagnosis, treatment, and Rev Obstet Gynecol. 2008;1(4):179–85.
20. Curcio AM, Shekhawat P, Reynolds AS, Thakur Neurologic infections during pregnancy. Handb Clin Neurol. 2020;172:79–104.
21. Adriani KS, Brouwer MC, van der Ende A, van de Beek D. Bacterial meningitis in pregnancy: report of six cases and review of the Clinical Microbiology and Infection. 2012;18(4):345–51.
22. Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, et Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Journal. 2018;16(1).
23. Horta-Baas G, Guerrero-Soto O, Barile-Fabris Central nervous system infection by Listeria monocytogenes in patients with systemic lupus erythematosus: analysis of 26 cases, including the report of a new case. Reumatologia clinica. 9(6):340– 7.
24. Hung J-J, Ou L-S, Lee W-I, Huang J-L. Central nervous system infections in patients with systemic lupus J Rheumatol. 2005;32(1):40–3.
25. van Veen KEB, Brouwer MC, van der Ende A, van de Beek D. Bacterial meningitis in diabetes patients: a population-based prospective Sci Rep. 2016;6:36996.
26. Carrillo-Esper R, Carrillo-Cordova LD, Espinoza de los Monteros-Estrada I, Rosales-Gutiérrez AO, Uribe M, Méndez-Sánchez Rhombencephalitis by Listeria monocytogenes in a cirrhotic patient: a case report and literature review. Ann Hepatol. 12(5):830– 3.
27. Mazzitelli M, Barone S, Greco G, Serapide F, Valentino P, Giancotti A, et al. Listeria infection after treatment with alemtuzumab: a case report and literature review. Would antibiotic prophylaxis be considered? Infez Med. 2020;28(2):258–62.
AMU 2022. Volumen 4, Número 1
Fecha de envío:
23/03/2022
Fecha de aceptación:
04/04/2022
Fecha de publicación:
31/05/2022