Guillermo Ramírez-García 1; Pablo García-Molina 2; María Consuelo Flor-Cremades 3; Beatriz Muñoz-Rojas 4; Javier Moleón-Moya 5
1 Master’s Degree in Anthropology and Forensic Genetics, University of Granada (UGR)
2 Master’s Degree in Biotechnology, University of Granada (UGR)
3 Faculty of Pharmacy, University of Granada (UGR)
4 Faculty of Medicine, University of Granada (UGR)
5 Department of Pharmacology, Faculty of Pharmacy, University of Granada (UGR)
Translated by:
Paula Asensi-Gómez 6; Ana Carbajo-García 7; Juan Luis Cobano-Jiménez 8; Elisa Lenker-Andrade 9; Iván Vázquez Delgado 9; Elena Vivas-del-Torno 6
6 Faculty of Human and Social Sciences, University of Comillas
7 Faculty of Human and Social Sciences, University Jaume I (UJI)
8 Faculty of Philosophy and Arts, University of Alcalá (UAH)
9 Faculty of Translation and Interpreting, University of Granada (UGR)
Objectives
The objective of this study is to evaluate the use of hydroxychloroquine and tocilizumab at the beginning of the COVID-19 pandemic and to describe the profile of patients who received these treatments.
Method
The medical records of 403 patients admitted for COVID-19 from March 1 to April 15, 2020 at the San Cecilio University Hospital (Granada, Spain) were analyzed. The data collected included sex, age, days hospitalized, previous pathologies and/or treatments, possible outcomes and drugs administered at the hospital. Student’s t-test and Pearson’s chi-square tests were used as statistical parameters to estimate the possible associations between the defined variables.
Results
Patients with a mean age of 66 years (standard deviation = 15.38), were hospitalized for an average of 15 days (standard deviation = 12.89). The ICU admission rate was 9.93 %, and the death rate added up to 17.37 % of the total number of patients. During the first wave of the pandemic, hydroxychloroquine was administered to the majority of hospitalized patients, while tocilizumab was restricted to the more severe cases.
Conclusions
The results showed two distinct trends in the use of the drugs studied. Tocilizumab was administered to a small number of patients, mainly those with longer length of stay or with complications. Hydroxychloroquine was administered independently of the initial characteristics of patients, especially those who presented comorbidities or took multiple medications.
Keywords: COVID-19, SARS-CoV-2, tocilizumab, hydroxychloroquine, hospitalization
Introduction
In December 2019, a case of atypical pneumonia of unknown origin was reported for the first time in Wuhan (China), which was later named as coronavirus disease 19 (COVID-19). The etiological agent of this pathology is the coronavirus responsible for the severe acute respiratory syndrome (SARS-CoV-2), which caused an unprecedented global pandemic with devastating consequences not only for public health, but for the economy (1). The main problem with SARS-CoV-2 was the combination of rapid spread and heterogeneous symptomatology that could lead to hospitalization or even death (2). Most patients diagnosed with COVID-19 were not severe cases, being asymptomatic or having common nonspecific symptoms such as fever, cough and myalgia (3). Nevertheless, those who developed complications like severe acute respiratory syndrome or infections were associated with increased admission to the ICU and high mortality (4). To reduce the morbidity and mortality rates associated with COVID-19, pharmacological treatment of patients infected with SARS-CoV-2 became a priority. At the beginning of the pandemic, a large number of therapeutic agents were tested in search of new indications for drugs already approved by international organisations, such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA), while awaiting the development of an effective and safe vaccine (5).
In Spain, the first wave of the pandemic lasted from the beginning of March until June 2020 (6). The main drugs used to treat the disease in hospitals and primary care centers were antimalarial medication (hydroxychloroquine [HCQ]), steroids (methylprednisolone) and antivirals (remdesivir), together with antibiotics in some cases. The use of interleukin 1 (IL-1) or 6 (IL-6) inhibitors, such as tocilizumab (TCZ), and Janus kinase inhibitors, such as ruxolitinib, is also worth mentioning. Several types of interferon (IFN), such as IFN-1b, were also used. Therefore, the trend during this period was the use of rather nonspecific agents, with the aim of avoiding the deterioration of the affected organs associated with the progression of the infection in patients (7). The combined administration of several drugs was also widely used, such as HCQ coupled with azithromycin in patients with comorbidities (8). In addition, nucleoside analogue antivirals, such as remdesivir, were used in high-risk groups. This reduced the viral load, but it did not considerably improve symptomatology (9).
Among the drugs with a new indication was HCQ stood out, an antimalarial drug also used to treat lupus and arthritis. The evidence supporting the indication for this drug was based on its used in previous SARS-CoV-1 outbreaks (11) and on the similarity of COVID-19 to other inflammatory pathologies. Initially, both HCQ and chloroquine (CQ) were mostly used in more severe cases (12). Another relevant drug during the pandemic was TCZ. At first, its used was avoided due to the uncertainties raised in the scientific literature (13). The studies that sought to determine the effectiveness of this drug against COVID-19 did not show significant variations with respect to other treatments (14, 15). However, as the pandemic progressed, the number of individuals receiving TCZ and the knowledge of this drug increased simultaneously (16-18).
As such, we set out to determine the role of these two drugs during the first wave of the pandemic and their association with other variables of interest. The objective of this longitudinal observational study was to evaluate the use of HCQ and TCZ during this period in Spain and ultimately to describe the profile of the patients who received these treatments.
Materials and methods
Design
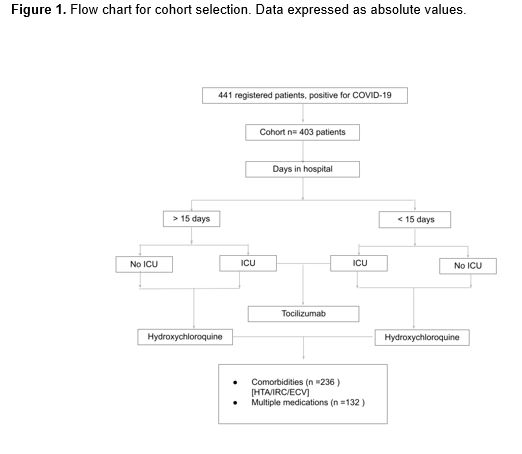
The planning and development of this longitudinal observational study was made following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The retrospective analysis was carried out on data provided by the San Cecilio University Hospital (Granada, Spain) and corresponds to 441 patients hospitalized for COVID-19 in this facility from March 1 to April 15, 2020. From the database provided, individuals with an incomplete medical history were discarded, resulting in a final sample of 403 patients. The registry of variables included baseline characteristics and possible outcomes, as well as drugs used for each patient (Figure 1).
Variables
All patient-related information was obtained from electronic medical records where the following variables were included:
- Patient’s general information: age, sex and country of origin.
- Hospitalization data: date of admission date to the center; date of discharge from the center or, otherwise, date of death; CURB-65 score; comorbidity; need for admission to the ICU and number of days hospitaliz
- Medical records of previous diseases: hypertension (HT), diabetes (DM), pneumological pathologies, chronic kidney failure (CKF), cardiovascular diseases (CVD), autoimmune diseases, neoplasms, asthma and chronic obstructive pulmonary disease (COPD). Also included in this section were current treatments associated with underlying pathologies, going from immunosuppressants to biologic drugs, as well as polypharmacy.
- Records of medication administered during hospitalization: HCQ, corticosteroids boluses (CTC), lopinavir/ritonavir, azithromycin, angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor antagonists (ARA2), antibiotics and TCZ. This section also included if the occurrence of cytokine release syndrome (CRS) or the need for cardiopulmonary resuscitation (CPR) when admitted to the hospital.
- Dependency and domiciliary records.
Statistical analysis
The statistical analysis of the data was made using two analysis software packages: IBM SPSS ® (V.26) and R (R Foundation for Statistical Computing, Wien, Austria), V.4.0.4. The relationship between the variables previously mentioned and the drugs of interest was evaluated using the following statistical parameters: Student’s t-tests and Pearson’s chi-square tests, with a 95 % confidence interval in all the analyses.
Ethical considerations
The provincial Research Ethics Committee of Granada approved this study on October 1, 2020. The database used was anonymized, in compliance with the Declaration of Helsinki.
Results
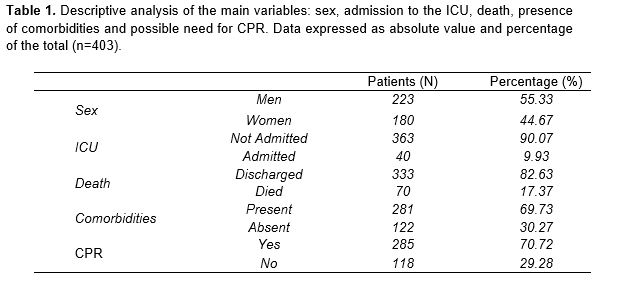
The study population consisted of 403 individuals, of whom 55.33 % were male and 44.67 % female. The mean age of the patients in the sample was 66 years, with a standard deviation (SD) of 15.38. The average length of hospitalization was 15 days (SD=12.89). Despite the high incidence of hospitalization, only 40 patients (9.9 %) required admission to the ICU. Similarly, only 17.4 % of the subjects had a fatal outcome, amounting to 70 deaths. It should be noted that 69.73 % of the patients had some previous pathology, and 285 of the total number of patients required CPR at the time of admission (Table 1).
Relationship between length of hospital stay and use of a given drug
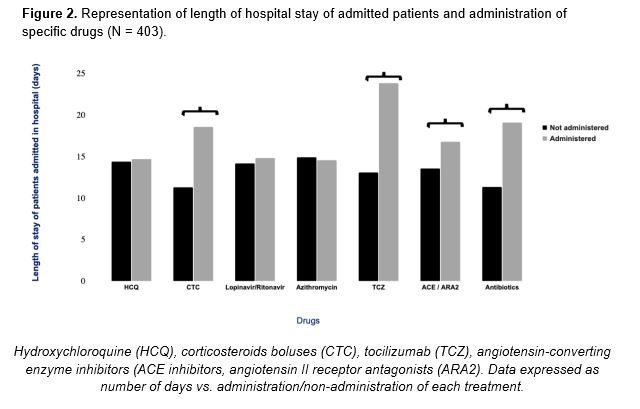
Before starting the in-depth analysis of the drugs under study, the relationship between the administration of a given drug and the number of days of hospitalization was determined. According to the results obtained, HCQ was used for a wide range of individuals, regardless of the number of days of hospitalization. In turn, TCZ, CTC, antibiotics and the ACE inhibitors/ARA2 combination were the preferred options for those patients who remained longer in hospital, as shown in Figure 2.
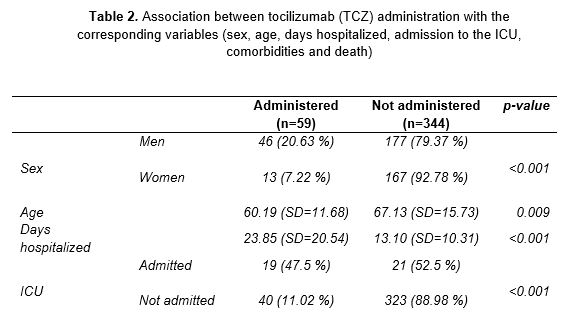
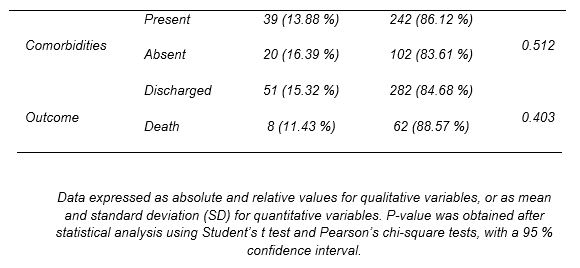
Tocilizumab
TCZ (Table 2) was administered to 59 patients (14.6 % of the total number of subjects). Data suggests an association with its use in men (p<0.001). A relationship was also established between TCZ use and age (p=0.009), with a mean value of 60 years (SD=11.68). Regarding the hospitalization period, patients treated with this drug remained hospitalized longer than those who did not receive it (p<0.001). In addition, the joint use of TCZ with other drugs of preferential use in long-stay patients was analyzed. As a result, an association was observed in the coadministration of the drug with antibiotics (p=0.006).
Since prolonged hospitalization could be related to the worsening of symptoms and the referral to intensive care, the correlation between the use of TCZ and admission to the ICU was studied. As expected, there was an association between drug administration and the need for intensive care (p<0.001). The majority of patients treated with TCZ required CPR at the time of admission (p<0.001). Similarly, 57 of the 59 patients who received the drug eventually presented CRS during hospitalization (p<0.001). Considering this finding, a relation in the joint use of TCZ and CTC was determined, with a p-value of <0.001.
Finally, other risk factors that could be involved in the worsening of the disease were analyzed. In contrast to previous results, it was not possible to relate TCZ use with the presence of comorbidities at the time admission to the hospital (p=0.512), nor was it associated with other drugs in patients taking multiple medications prior hospitalization (p=0.388).
Hydroxychloroquine
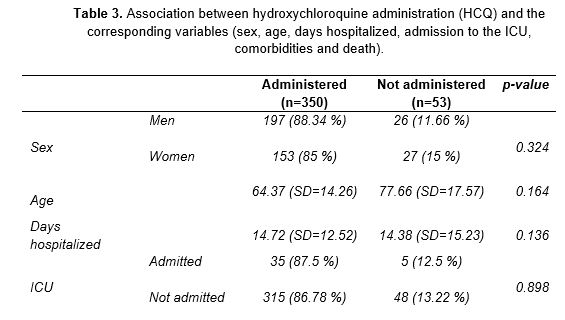
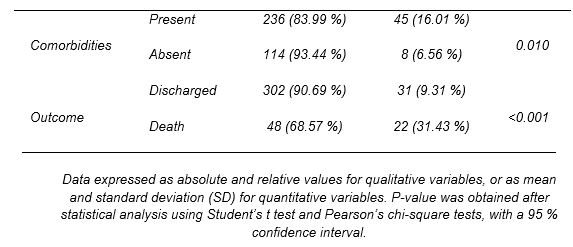
The other drug under study was HCQ. It was administered to 350 out of the 403 patients, that is, 86.8% of the patients admitted for COVID-19. This group comprised 197 males and 153 females. The statistical analysis performed found no association between sex and drug administration (p=0.324), showing that HCQ was used irrespective of sex (Table 3). Despite its extensive use, HCQ failed to significantly reduce the length of hospital stay (p=0.136). The average number of days hospitalized was similar in those treated and not treated with HCQ. In addition, those who were eventually admitted to the ICU were treated with HCQ in a similar proportion to those who remained on the ward. No direct association was established between referral to intensive care and the use of this drug (p=0.898).
Unlike the previous variables, an association was found between comorbidity and HCQ use (p=0.01). Considering this finding, the relationship between HCQ use and patients with previous at the time of admission to the hospital was also tested. A correlation was observed between individuals who received the drug and who presented HT (p=0.003), CKF (p=0.002) or CVD (p<0.001). Accordingly, patients who needed CPR at the time of admission received HCQ in a higher proportion than the rest of patients (p<0.001). Patients with comorbidities required specific treatments for their pathologies. Therefore, the relationship between drug administration and multiple medications was tested, with a p-value of 0.002.
Finally, the correlation between HCQ and the use of other therapeutic agents administered in the hospital was analyzed. An association was found between in the joint use of HCQ and CTC (p=0.002), azithromycin (p<0.001) and lopinavir/ritonavir (p<0.001), which were used regardless of the number of days of hospitalization.
Discussion
Relationship between length of hospital stay and use of a given drug
Based on the results, it was observed that TCZ, CTCs, ACE /ARA2 inhibitors and antibiotics were preferentially used in patients with a longer hospital stay. The choice of these drugs was based on the improvement of symtoms. TCZ is an agent that blocks the binding of IL-6 to its receptor, leading to a decrease in the effects of this proinflammatory cytokine (19). CTCs, especially glucocorticoids, were also administered due to their ability to reduce the CRS associated with COVID-19 in patients who manifest hemophagocytic syndrome (HPS) and macrophage activation syndrome (MAS) (16). Regarding the use of ACE/ARA2 inhibitors, there were no standardized criteria, as the interaction of SARS-CoV-2 with the Renin-Angiotensin-Aldosterone system was not fully clear. For this reason, although some authors rejected the use of these drugs in COVID-19 patients (20), scientific evidence proofs HT treatment a priority (20, 21). Finally, antibiotic treatment was reserved for patients who presented nonspecific symptoms not associated with the disease or patients with complications (22).
Tocilizumab
The data obtained from analysing TCZ showed a trend in the use of this drug in patients with a longer hospital stay and consequent referral to the ICU. In fact, a relevant association was found in the joint use of TCZ and antibiotics. These antimicrobial agents were administered to a greater extent to individuals hospitalized for extended periods, as observed previously (15).
Regarding the use of TCZ in the ICU, admission to intensive care was associated with a worsening of the disease. This description matched the findings of other studies in which patients who received TCZ presented poorer health and old age (23,24). Requiring CPR at admission served as an indicator of patients at higher risk of severe COVID-19. The fact that TCZ was preferentially used for this group of patients supports what was observed in previous studies (25).
In addition, TCZ was used almost exclusively for individuals who presented CRS at any time during hospitalization. Given this information, an association was found between the use of the drug in conjunction with CTC, which were widely indicated to treat this complication (16). However, when evaluating the coadministration of TCZ with other drugs in the patients under study, no correlation could be established. The explanation for this could be owed to the restricted use of the drug, as there is no evidence of contraindications in the joint use of TCZ and other therapeutic agents (26, 27, 28).
With all the information obtained from the analysis, it was possible to preliminarily describe the TCZ target patient in the cohort studied. Generic features included a mean age of 60 years, extended hospital stay associated with ICU admission or worsening of the disease, and the occurrence of CRS.
Hydroxychloroquine
The results regarding HCQ showed a wide use of the drug regardless of the baseline characteristics of the patients. This trend was common during the beginning of the pandemic and was based on recommendations that positioned it as one of the best choices against SARS-CoV-2 (29).
Regarding the most relevant associations, a correlation was observed between the presence of two or more diseases at the time of admission and the indication for HCQ. When examining the pathologies included among comorbidities, an association was found between the drug and HT, CVD and CKD. As described by other authors (30), both HT and the complications derived from it, which constitute the majority of CVD cases, require chronic treatment. Therefore, patients with these pathologies generally receive multiple medications.
The relationship between subjects receiving multiple medications and the administration of HCQ was tested and a relevant association was found. The reason for this result could be the widespread use of the drug during the first wave of the pandemic (12) rather than the protective effects on this group of patients. Nonetheless, a correlation was observed between the joint administration of HCQ and azithromycin, an effective combination supported by scientific evidence (31).
The data obtained from the analysis enables the profiling of patients who received HCQ during hospitalization. The general characteristics of these subjects were: a mean age of 64 years, generally presenting pathologies such as HT, CKD and CVD, and, to a lesser extent, patients receiving multiple medications.
Limitations
The study has certain limitations inherent to its observational nature. Other than these, the small sample size (n=403) due to exclusion criteria and the short period of data collection are worth noting. Although these characteristics could imply a possible imprecision bias with regard to the generalization of the results, the strength of the statistical parameters used increases their validity (7).
Conclusions
The results showed two totally different trends in the use of the drugs under study. On the one hand, TCZ was administered to a very small number of the total number of subjects. It was mainly indicated for those who remained hospitalized longer or whose clinical conditions worsened. Its use was also associated with ICU patients in coadministration with antibiotics. On the other hand, HCQ became one of the most widely used therapeutic agents regardless of the initial characteristics of the patients. It became particularly relevant for patients with comorbidities or receiving multiple medications. In most severe cases, HCQ was used in conjunction with azithromycin.
Statements
Acknowledgements
The authors of this paper would like to thank the involvement of the coordinating and teaching staff of the “Producción y traducción de artículos científicos biomédicos (III ed.)” and the “Traducción inversa de artículos científicos biomédicos (español-inglés)” courses, as well as the English translation team. Likewise, the authors acknowledge and thank the healthcare professionals who worked during the pandemic at the Hospital Universitario San Cecilio (Granada) for their invaluable work and admirable commitment.
Conflicts of interest
The authors of this paper declare no conflicts of interest.
References
- Lemmon ME, Chapman I, Malcolm W, Kelley K, Shaw RJ, Milazzo A, et al. Beyond the First Wave: Consequences of COVID-19 on High-Risk Infants and Families. Am J Perinatol. 2020;37(12):1283.
- Awadasseid A, Wu Y, Tanaka Y, Zhang W. Initial success in the identification and management of the coronavirus disease 2019 (COVID-19) indicates human-to-human transmission in Wuhan, China. Int J Biol Sci. 2020;16(11):1846.
- Cheong J, Bartell N, Peeraphatdit T, Mosli M, Al-Judaibi B. Gastrointestinal and liver manifestations of COVID-19. Saudi J Gastroenterol Off J Saudi Gastroenterol Assoc. 2020;26(5):226.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- de Brouwer R, van Veldhuisen DJ, de Boer RA. Surviving the first COVID-19 wave and learning lessons for the second. Eur J Heart Fail. 2020.
- Gallego VM, Codorniu JM, Cabrero GR. The impact of COVID-19 on the elderly dependent population in Spain with special reference to the residential care sector. Ciência & Saúde Coletiva. 2021;26:159–68.
- Gutiérrez-Abejón E, Tamayo E, Martin-Garcia D, Álvarez FJ, Herrera-Gómez F. Clinical Profile, Treatment and Predictors during the First COVID-19 Wave: A Population-Based Registry Analysis from Castile and Leon Hospitals. Int J Environ Res Public Health. 2020;17(24):9360.
- Risch HA. Early outpatient treatment of symptomatic, high-risk COVID-19 patients that should be ramped up immediately as key to the pandemic crisis. Am J Epidemiol. 2020;189(11):1218–26.
- Dubert M, Visseaux B, Isernia V, Bouadma L, Deconinck L, Patrier J, et al. Case report study of the first five COVID-19 patients treated with remdesivir in France. Int J Infect Dis. 2020;98:290–3.
- Bansal P, Goyal A, Cusick IV A, Lahan S, Dhaliwal HS, Bhyan P, et al. Hydroxychloroquine: A comprehensive review and its controversial role in coronavirus disease 2019. Ann Med. 2021;53(1):117–34.
- Ibáñez S, Martinez O, Valenzuela F, Silva F, Valenzuela O. Hydroxychloroquine and chloroquine in COVID-19: should they be used as standard therapy? Clin Rheumatol. 2020;39:2461–5.
- Pereira BB. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J Toxicol Environ Heal Part B. 2020;23(4):177–81.
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–5.
- Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin Exp Rheumatol. 2020;38(3):529–32.
- Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9.
- Rubio JLC, Del Castillo J de DL, de la Hera Fernández J, Arrabal EG, Ruiz MC, Centeno NO. Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection. Med Clínica (English Ed. 2020;155(4):159–61.
- Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–5.
- Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44.
- Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–8.
- Arcos FS, Puche AR, Vera TV. Controversy regarding ACE inhibitors/ARBs in Covid-19. Rev Esp Cardiol (Engl Ed). 2020;73(6):516.
- Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Oxford University Press US; 2020.
- Miranda C, Silva V, Capita R, Alonso-Calleja C, Igrejas G, Poeta P. Implications of antibiotics use during the COVID-19 pandemic: present and future. J Antimicrob Chemother. 2020;75(12):3413–6.
- Klopfenstein T, Zayet S, Lohse A, Balblanc J-C, Badie J, Royer P-Y, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020;50(5):397–400.
- Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51.
- Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8(5):695.
- Samaee H, Mohsenzadegan M, Ala S, Maroufi SS, Moradimajd P. Tocilizumab for treatment patients with COVID-19: Recommended medication for novel disease. Int Immunopharmacol. 2020;107018.
- Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–5.
- Fontana F, Alfano G, Mori G, Amurri A, Tei L, Ballestri M, et al. Covid-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. 2020;20(7):1902–6.
- Zhou D, Dai S-M, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75(7):1667–70.
- Fravel MA, Ernst M. Drug Interactions with Antihypertensives. Curr Hypertens Rep. 2021;23(3):1–8.
31. Gautret P, Lagier J-C, Parola P, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
AMU 2021. Volumen 3, Número 1
Fecha de envío:
14/03/2021
Fecha de aceptación:
04/04/2021
Fecha de publicación:
31/05/2021