Celia Pérez-Díaz 1; Marta Miranda-García 2; Maribel González-Acedo 1
1 Master’s Degree in Research and Advances in Preventive Medicine and Public Health, University of Granada (UGR)
2 Faculty of Pharmacy, University of Granada (UGR)
Translated by:
Marina Acién-Martín 3; Jacqueline González-del-Pino 3; Lidia Guerrero-Díaz 4; Nelly Paniagua-Becerra 5; Mª Carmen PradosCastilla 6; Andrea Sánchez-Molina 3
3 Faculty of Translation and Interpreting, University of Granada (UGR)
4 Faculty of Philosophy and Letters, University of Málaga (UMA)
5 Lima Institute of Technical Studies (LITS)
6 Faculty of Philosophy and Letters, University of Granada (UGR)
Bisphenol A belongs to a group of environmental pollutants, called endocrine disruptors, whose relationship with various health disorders is becoming increasingly evident. One of these disorders is polycystic ovary syndrome (PCOS). This narrative review attempts to define key aspects of both bisphenol A and PCOS, as well as to consolidate the current knowledge about them with the aim to establish a solid basis for their relationship and implication in health. Different studies point out a possible relationship not only between PCOS and exposure to bisphenol A, but also to other diseases that are more prevalent in women with this syndrome, such as disorders in hormone regulation or metabolism. It is necessary, however, to deepen the knowledge about the relationship between bisphenol A and PCOS in order to provide more evidence. To this effect, it would be interesting to develop different epidemiological studies focused on strengthening and reaffirming the potential causal association.
Keywords: bisphenol A, polycystic ovary syndrome, PCOS, endocrine disruptor, reproductive health.
Introduction
Endocrine disruptors belong to a heterogeneous group of molecules (natural or synthetic) which can interfere with the endocrine system. This is because their phenolic structure can mimic or antagonize endogenous steroid hormones (1) and cause different metabolic disorders, even the development of hormone-dependent cancers (2).
Bisphenol A is one of the endocrine disruptors most frequently found in the environment (3). This is mainly due to its increasing industrial use in the production of many synthetic materials or polycarbonates, among others (4). It is considered a ubiquitous compound that can be found in reusable bottles, plastic and metal items used in food packaging, toys, medical equipment or even dental compounds. In this regard, several studies reported that it is present in the air, dust or water, with ingestion of contaminated food and water being the main route of exposure (5). However, absorption through the skin and respiratory system can also occur (6). In this line, studies developed by the Center for Disease Control and Prevention showed a detectable level of bisphenol A in samples of urine, amniotic fluid, placental tissue, umbilical cord and fetal serum (4). This compound has been detected in follicular fluid, suggesting that oocytes would have been exposed to this contaminant agent during the folliculogenesis (1).
On the other hand, PCOS is characterized by a heterogeneous presence of anovulation, hyperandrogenism, polycystic ovarian morphology, metabolic dysfunction and infertility (7). In addition, this syndrome is associated with sexual and psychological problems (8). Thus, it increases the probability of having symptoms of depression and/or anxiety (9).
Globally, it is considered the most common and heterogeneous endocrine disorder in women of child-bearing age (10). Its prevalence is between 6% and 21% depending on the diagnostic criteria (11). In addition, more than 75% of cases of anovulatory infertility are ascribed to PCOS (12).
Although the pathophysiology of the syndrome is not clear, an increased prevalence of reproductive diseases, a decreased female fertility in recent years, and a strong presence in our environment of pollutants capable of altering the proper function of the endocrine system, make the role of the environment increasingly important (13). Hence, a need arises for clarifying the potential relationship between endocrine disruptors and the potential reproductive disorders that could result from such exposure (3). Therefore, the objective of this narrative review is to study the relationship between bisphenol A exposure and PCOS because of the growing interest in this topic.
What do we know about bisphenol A?
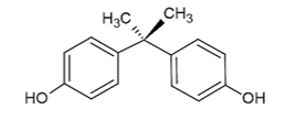
Bisphenol A (Figure 1) is an industrial compound able to mimic the effect of estrogenic hormones. It is an endocrine disruptor widely used in the manufacture of many consumer goods (14). Endocrine disruptors are agents that interfere with the synthesis, secretion, transport, binding, action or elimination of the body’s natural hormones responsible for the maintenance of homeostasis, reproduction, development and/or behavior (15). Additionally, due to the effects shown by bisphenol A in changing several metabolic pathways in humans, particularly those related to hormonal regulation of reproductive processes, bisphenol A is considered a xenoestrogen. Xenoestrogens include compounds that modify the synthesis, transport, activity and metabolism of endogenous estrogens. Therefore, they affect the growth, development and reproduction of organisms (4, 16).
Figure 1. Molecular structure of bisphenol A
Bisphenol A was first synthesized by Alexander Pavlovich Dianin in 1891. It was recognized as an artificial estrogen by the British chemist Charles Edward Dodds in 1930. Shortly thereafter, it was tested for the prevention of adverse pregnancy outcomes in women with a history of miscarriage. It was not until 1947 when the Food & Drug Administration (FDA) approved diethylstilbestrol (2). Subsequently, the industrial use of Bisphenol A began in the 1950s. In 2008 its production was estimated at about 5.2 million tons worldwide and reached 7.7 million tons in 2015. The consumption is estimated to increase to 10.6 million tons in 2022 (6).
Although the safety of bisphenol A in consumer goods is largely guaranteed, several studies conducted over the last twenty years claim that this compound is not only widely distributed in the environment, but also exhibits toxicity even at low doses (4). This is because adverse reactions in humans generally result from chronic exposure (5).
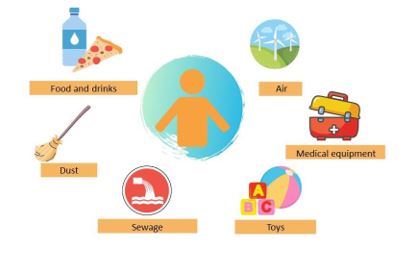
This pollutant is commonly found in urine and blood samples, confirming the high exposure to these chemicals (3). Oral ingestion is the main source of exposure in humans, although there are many routes of exposure (Figure 2). Evidence has been found that bisphenol A can enter food during the storage time by prolonged contact with plastic, paper containers or even cans. In addition, processes such as washing and heating can stimulate its release, increasing its concentration in food. It has also been shown that bisphenol A can be released from feeding bottle materials (4).
Figure 2. Sources of bisphenol A exposure
These circumstances have encouraged the implementation of numerous restrictive policies, such as Directive 2011/8/EU. This directive prohibits the use of bisphenol A in feeding bottles due to the lack of knowledge about its adverse effects on the biochemical changes in the brain, the immunomodulatory activity, and the risk of developing breast cancer (4). In addition, the recommended daily intake in Europe has decreased from 50 mg/kg bw/day to 4 mg/kg bw/day (6).
Since bisphenol A can interact with estrogen receptors, it can interfere with fertility in women (2) by modifying steroidogenesis, folliculogenesis and ovarian morphology (3). Furthermore, its exposure could be related to alterations in oocyte production and adverse effects on human fertility by disrupting the synthesis of sex steroid activity (4). Bisphenol A is also associated with metabolic diseases such as type 2 diabetes, reduced insulin sensitivity, alterations in glucose and lipid metabolism, and increased risk of cardiovascular disease, among others (5).
What do we know about PCOS?
PCOS was first described at the beginning of the 20th century by Lesnoy in 1928 and by Stein and Leventhal in 1935 (17). The diagnostic criteria have been modified over time. Moreover, this syndrome has become important after the international conference on PCOS promoted by the National Institutes of Health (NIH) in 1990. It was in 2003 when these criteria were standardized to the Rotterdam criteria for the diagnosis of PCOS (18).
Another important contribution to the syndrome’s definition are the Androgen Excess and PCOS Society’s (AE-PCOS) criteria from 2006 (19). There was an attempt to settle and unify the observations relevant to the disease on the Evidence-based Methodology Workshop on Polycystic Ovary Syndrome from 2012. However, the results did not have a great impact. Nowadays, Rotterdam criteria are the most widely accepted (20, 21).
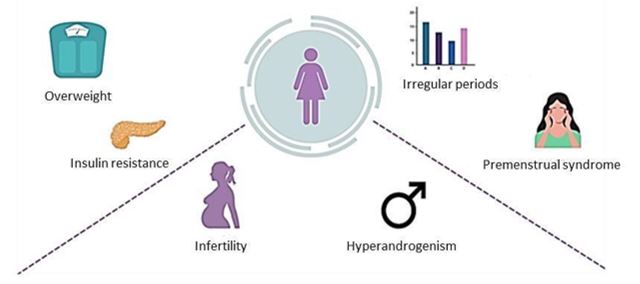
PCOS is characterized by a number of signs and symptoms (Figure 3). The most frequent are related to excessive androgen production, including hirsutism, alopecia and acne. Hair growth is particularly typical on the chin, neck, lower face and preauricular area. Excessive hair growth usually occurs on the lower back, abdomen, buttocks, perineal area, inner thighs, and periareolar area. Ovulatory dysfunction, irregular periods and fertility problems are common in women with PCOS (22). Insulin resistance and polycystic ovarian morphology can also be part of the manifestation of PCOS, although they are not strictly necessary for a diagnosis. Weight gain and insulin resistance are very common, varying the prevalence of insulin resistance between 40% and 70% when using alternative markers (23). The examination of ovarian morphology is performed by ultrasonography, which will confirm polycystic ovarian morphology when the volume of an ovary is ≥ 10 ml and/or there is an increase in antral follicles (7).
Figure 3. PCOS signs and symptoms
An agreement between the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) was promoted as a possible solution to the difficulties for the standardization of diagnostic criteria. According to the agreement, PCOS can be diagnosed when two of the three following criteria are met: i) clinical or biochemical hyperandrogenism; ii) chronic oligomenorrhea and/or anovulation; iii) presence of polycystic ovaries in transvaginal ultrasound scan. These are known as the Rotterdam criteria for PCOS diagnosis. There are currently four recognized PCOS phenotypes, which are defined by different combinations of the syndrome’s features. Phenotype 1, also called “complete” PCOS phenotype, includes hyperandrogenism, primary ovarian insufficiency (POI), and polycystic ovarian morphology. In the rest of the phenotypes, however, only two of the three criteria are present: phenotype 2 includes hyperandrogenism and POI; phenotype 3, hyperandrogenism and polycystic ovarian morphology; and phenotype 4, POI and polycystic ovarian morphology (7).
In addition to its own characteristics, PCOS is related to decreased fertility and complications during pregnancy, obesity and dyslipidemia, insulin resistance and type 2 diabetes, metabolic syndrome, higher risk of anxiety and depression, higher risk of cardiovascular disease (10), and higher risk of endometrial hyperplasia and carcinoma (7).
Despite its unclear pathophysiology, growing evidence suggests that PCOS might be a complex multigenetic disorder with strong epigenetic and environmental influences (21). Patients with PCOS showed alterations in their hypothalamic–pituitary axis and follicle sensitivity to hormones, insulin resistance and adipocyte dysfunction (7). PCOS can be understood as a state of chronic inflammation at a low level, which makes it necessary to adopt a lifestyle avoiding oxidative stress, acidosis or immune activation in order to alleviate it (24).
Bisphenol A and Polycystic Ovarian Syndrome. What is the evidence?
Since bisphenol A can interact with the endocrine system, many research studies have tried to describe its relationship with PCOS. The aim was to discern the role that bisphenol A plays in PCOS etiology. Both in vivo and in vitro experiments (4, 25), as well as transversal and longitudinal epidemiological studies, have been conducted to study this topic (3, 26, 27).
As for the results provided by the epidemiological studies, the wide presence of bisphenol A in the organism has been observed, being higher in women diagnosed with PCOS (3, 26, 27). Based on this evidence, the previous exposure to environmental pollutants that act as endocrine disruptors could be a risk factor for developing this syndrome (27). Therefore, in the cross-sectional study conducted by Zhou et al., a mean concentration of 2.35 ng/ml was detected in the urine of women with PCOS, being a higher level than the one recorded in other women without this pathology (1). Accordingly, other authors pointed out how, when compared to the healthy controls, the presence of serum bisphenol A is significantly larger in women with PCOS (28).
These studies have not only documented the widespread presence of bisphenol A in the organism, but also a significant decrease in the antral follicle count, along with lower levels of the anti-Müllerian hormone and the follicle stimulating hormone. This was associated with a relative decrease in the ovarian reserve of the women in the sample (1). Another consequence is hyperandrogenism, positively correlated with serum total testosterone levels and the free androgen index (28, 29). This could be related to the stimulation of the cell structure surrounding the follicular antrum. The 17-α-hydroxylase enzyme, key in the gonadal steroidogenesis, is deregulated (29). However, other studies suggest that these alterations, characteristic of PCOS, can also be the result of the exposure to other environmental pollutants such as brominated diphenyl ethers, organochlorine pesticides, perfluorinated compounds or phthalates (27).
Regarding the rest of the criteria for PCOS diagnosis, other cross-sectional studies have shown a significantly larger waist-to-height ratio in women with higher bisphenol A levels in their urine. This justifies a possible tendency to be overweight, insulin level above the reference limit (24.9 mIU/L) and a greater resistance to insulin evidenced by the homeostasis model assessment (HOMA-IR) (5, 29). This same population shows an alteration of the lipid profile with moderately elevated total cholesterol and triglycerides levels, along with a decrease of the high-density lipoprotein (HDL) compared to controls. Moreover, an increase in the leptin levels was observed. This increment of the hormone responsible for regulating the appetite may explain weight gain in women diagnosed with PCOS (5).
In other controlled trials in laboratory, such as in vivo and in vitro experiments (4, 25), reproductive alterations related to PCOS have also been observed. Irregular periods, alterations in the ovarian development with a decrease in corpus luteum and antral follicles, in addition to an increase in atretic follicles and the presence of cysts that give name to this pathology, have been observed (4, 25).
Conclusion
Bisphenol A is a pollutant that is on the rise in the industry and seems to have a direct correlation to reproductive health. At the same time, PCOS has become an increasingly prevalent disease. In general, the literature included in this revision suggests that bisphenol A may exacerbate the metabolic risk in women with PCOS or even be part of their etiology due to its ability to interfere with the endocrine system. Furthermore, bisphenol A would enhance weight gain, hyperinsulinemia and insulin resistance, along with dyslipidemia and hyperandrogenism. Due to the lack of studies, it is essential to further analyze the impact that this compound could have on reproductive health, particularly on PCOS. This could result in the implementation of future restrictions on its industrial use, thus reducing its presence on an environmental level. Consequently, it would be appropriate to carry out further epidemiological studies to assess the impact to a long-term bisphenol A exposure, enabling the establishment of a more solid causal association.
Statements
Acknowledgements
The authors of this paper would like to thank the involvement of the coordinating and teaching staff of the “Producción y traducción de artículos científicos biomédicos (III ed.)” and the “Traducción inversa de artículos científicos biomédicos (español-inglés)” courses, as well as the English translation team.
Conflicts of interest
The authors of this paper declare no conflicts of interest.
References
- Zhou W, Fang F, Zhu W, Chen ZJ, Du Y, Zhang J. Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. Int J Environ Res Public Health. 2016;14(1):1-7.
- Rutkowska A, Rachon D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2014;30(4):260-5.
- Akgul S, Sur U, Duzceker Y, Balci A, Kizilkan MP, Kanbur N, et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(12):1084-7.
- Tomza-Marciniak A, Stepkowska P, Kuba J, Pilarczyk B. Effect of bisphenol A on reproductive processes: A review of in vitro, in vivo and epidemiological studies. J Appl Toxicol. 2018;38(1):51-80.
- Milanovic M, Milosevic N, Sudji J, Stojanoski S, Atanackovic Krstonosic M, Bjelica A, et al. Can environmental pollutant bisphenol A increase metabolic risk in polycystic ovary syndrome? Clin Chim Acta. 2020;507:257-63.
- Jurewicz J, Majewska J, Berg A, Owczarek K, Zajdel R, Kaleta D, et al. Serum bisphenol A analogues in women diagnosed with the polycystic ovary syndrome – is there an association? Environ Pollut. 2021;272(115962):1-7.
- Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol. 2018;132(2):321-36.
- Eftekhar T, Sohrabvand F, Zabandan N, Shariat M, Haghollahi F, Ghahghaei-Nezamabadi A. Sexual dysfunction in patients with polycystic ovary syndrome and its affected domains. Iran J Reprod Med. 2014;12(8):539-46.
- Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075-91.
- Louwers YV, Laven JSE. Characteristics of polycystic ovary syndrome throughout life. Ther Adv Reprod Health. 2020;14:1-9.
- Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J Womens Health (Larchmt). 2015;24(4):299-307.
- Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2017;114(16):E3334-E43.
- Huo X, Chen D, He Y, Zhu W, Zhou W, Zhang J. Bisphenol-A and Female Infertility: A Possible Role of Gene-Environment Interactions. Int J Environ Res Public Health. 2015;12(9):11101-16.
- Wang Y, Zhu Q, Dang X, He Y, Li X, Sun Y. Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecol Endocrinol. 2017;33(1):21-5.
- Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118(9):1217-22.
- Rashidi B, Amanlou M, Lak T, Ghazizadeh M, Haghollahi F, Bagheri M, et al. The Association Between Bisphenol A and Polycystic Ovarian Syndrome: A Case-Control Study. Acta Med Iran. 2017;55(12):759-64.
- Rodgers RJ, Suturina L, Lizneva D, Davies MJ, Hummitzsch K, Irving-Rodgers HF, et al. Is polycystic ovary syndrome a 20th Century phenomenon? Med Hypotheses. 2019;124:31-4.
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. FertilSteril. 2004;81(1):19-25.
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91(11):4237-45.
- NIH. Evidence-based Methodology Workshop on Polycystic Ovary Syndrome (PCOS) 2012 [Last access: 21/11/2012]. Available at: https://www.nichd.nih.gov/newsroom/resources/spotlight/112112-pcos.
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270-84.
- Meier RK. Polycystic Ovary Syndrome. Nurs Clin North Am. 2018;53(3):407-20.
- Macut D, Bjekic-Macut J, Rahelic D, Doknic M. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract. 2017;130:163-70.
- Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27-36.
- Yang Z, Shi J, Guo Z, Chen M, Wang C, He C, et al. A pilot study on polycystic ovarian syndrome caused by neonatal exposure to tributyltin and bisphenol A in rats. Chemosphere. 2019;231:151-60.
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab. 2011;96(3):E480-E4.
- Vagi SJ, Azziz-Baumgartner E, Sjodin A, Calafat AM, Dumesic D, Gonzalez L, et al. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case-control study. BMC Endocr Disord. 2014;14(86):1-12.
- Konieczna A, Rachon D, Owczarek K, Kubica P, Kowalewska A, Kudlak B, et al. Serum bisphenol A concentrations correlate with serum testosterone levels in women with polycystic ovary syndrome. Reprod Toxicol. 2018;82:32-7.
- Akin L, Kendirci M, Narin F, Kurtoglu S, Saraymen R, Kondolot M, et al. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatr. 2015;104(4):e171-e7.
AMU 2021. Volumen 3, Número 1
Fecha de envío:
14/03/2021
Fecha de aceptación:
04/04/2021
Fecha de publicación:
31/05/2021