José Mateos-Granados ¹ , Carmen María López-Pérez ¹ , Ana Elena Lizana-Serrano ¹ , Álvaro Díaz Gómez ¹ , Alejandra Díaz-García ¹ , Raquel Moya-Barquero ¹
1 Estudiante del Grado en Medicina de la Universidad de Granada (UGR)
TRANSLATED BY:
Paola Rodríguez-González ² , José Luis Castillo-del-Águila ² , Irene Torres-Martínez ² , Ana García-Canteli ² , María Ruiz- Escrivá ² , Nuria Vadillo-Ucea ²
2 Student of the BA in Translation and Interpreting at the University of Granada (UGR)
Asbestos has been a source of concern in the health field since the first cases of cancerous disease were discovered. It has been used in roofing and building insultation, and for years the population has been exposed to its harmful effect. Nowadays, the scientific community is fully aware of the adverse effects of asbestos on the respiratory system. However, the consequences in other systems are not so clearly defined. In this review, we attempt to collect all published and studied information on the relationship between asbestos and gastro-intestinal (GI) cancer. For this purpose, we address separately each part of the digestive system in which possible evidence has been studied, as well as the generalities found in the scientific
literature on this relationship.
Keywords: asbestos, cancer, digestive system.
1. Introduction
Asbestos is a material classified as natural fibrous silicate mineral disposed in fibers. It has many physicochemical properties, including flexibility and resistance to high temperatures and exposure to chemicals. Because of these properties, it has been used in construction and in the insulation of houses, schools, and all types of buildings. Asbestos are divided into two groups: amphibole and serpentine asbestos. Amphiboles, such as crocidolite or blue asbestos are straight fibers. Other examples include amosite, anthophyllite or tremolite. Serpentine asbestos, chrysotile or white asbestos is made up of curved fibers and constitutes 95% of the asbestos used for building materials.
The use of asbestos in the constructions industry dates back to 1850. By the mid-20th century there was already evidence of the adverse health effects of this material. Nowadays, cases of patients affected by asbestos continue to be found despite the ban on its use in approximately 50 countries (1). Despite continuous and repeated warnings about the toxicity and carcinogenicity of asbestos-containing materials, a large number of people of all ages, including young children, are potentially exposed to asbestos (2). Furthermore, it has been demonstrated that exposure to these fibers has negative effects on the lungs, causing pleural mesothelioma, pulmonary fibrosis and bronchial carcinoma, among other diseases.
- Action mechanisms and exposure routes
The mechanisms by which exposure to asbestos may influence the risk of cancer are not well established. However, the ongoing presence of asbestos fibers on tissues is thought to cause an inflammatory effect. Properties of asbestos, such as the length and diameter of the fiber, its surface and its durability, are also thought to have an influence. Crocidolite has the smallest diameter and is considered the most harmful.
The possibility of asbestos producing one or the other pathology, depending on the access route into the organism, is currently being studied. Therefore, when inhaled, it produces lung disease; whereas, when ingesting its fibers, it may cause gastro-intestinal (GI) cancer. The most likely route of exposure involved in GI disorders is the intake of contaminated drinking water due to the large number of buildings with asbestos-cement pipes (3) or natural contamination.
- Asbestos and drinking water
Asbestos has been classified as a carcinogenic agent that can induce histological alteration in the GI tract and have negative effects in humans at a molecular level. It has been observed that there are around 7 million asbestos fibers per liter in water, being this contamination higher in surface water than in well water. These fibers come mainly from the deterioration or decomposition of asbestos-containing materials. These include wastewater from mining and other industries, or asbestos-cement pipes and water tanks still present in drinking-water supplies (4, 5).
However, no guideline value has yet been established for asbestos in drinking water (6), nor have restrictive limits been set on the concentration of fibers in water. This is due to the fact that the threshold of carcinogenic risk in the GI tract is still unknown. Moreover, there are many confounding factors which derive mainly from the difficult quantification of ingested fibers. (7).
Additionally, the effect of ingested asbestos may differ depending on the age group. Very little research has been conducted on this matter and it would be highly significant. Children are more susceptible than adults to environmental hazards due to their longer life expectancy, and living in a continuously contaminated geographical area results in longer exposure to orally ingested asbestos. Furthermore, children drink approximately 7 times more water than adults.
On the other hand, the mother may transfer asbestos fibers to the fetus (8). Asbestos fibers have been found in the placenta, lung, muscle, and liver, after performing an autopsy on stillborn babies, being the fiber count higher in the liver. In addition, the mean length of the fibers was similar to that of the fibers found in asbestos-cement pipes and cisterns.
Therefore, it is necessary to establish a maximum acceptable level of asbestos in drinking water all over the world. This would justify a review of the existing standards, in order to avoid an increased risk of developing cancer.
- Peritoneal neoplasms and other possible diseases
Scientific literature seems to support a strong association between exposure to asbestos and peritoneal neoplasms, whose current treatment options are unsatisfactory (9). It was found that workers exposed to chrysotile had lower risk than workers exposed to a mix of chrysotile and crocidolite. For this reason, the type of fibers is related to the location and, possibly, the severity of neoplasms, assuming that exposure to amphibole increases the risk of developing peritoneal tumors (10). The risk is proportional to the quantity of the substance and the exposure to it.
The size of the fibers seems to an important factor in the carcinogenic effect of asbestos. In a study where 168 cases of mesothelioma were analyzed, the majority of the fibers were no longer than 5 microns. No mechanism is known for the direct contact of asbestos with the peritoneum. The activation of signaling pathways in the lung, particularly those in which TFG-beta is involved, may be responsible for the development of peritoneal disease.
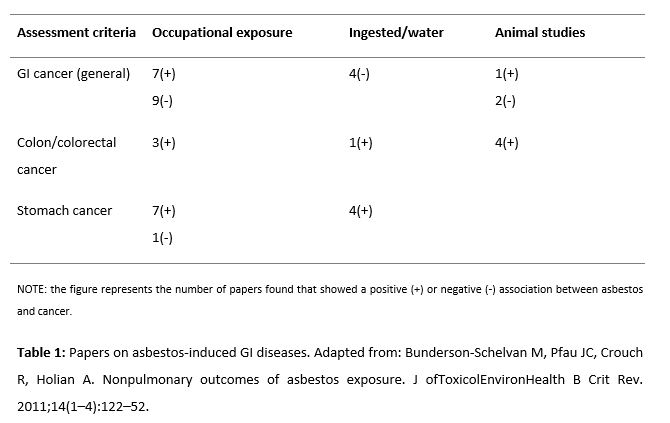
Moreover, it has been found that iron excess increases the carcinogenic potential of crocidolite due to an increase in oxidative stress. In fact, it is thought that free radicals are partially responsible for the mutagenic potential of asbestos as its mutagenicity is reduced by antioxidants (11). The adverse effects of asbestos are not confined to the respiratory system but also include ovarian cancer, GI cancer, brain tumors, blood disorder and peritoneal fibrosis. Finally, with regard to the digestive system, it is remarkable that despite the fact that the GI tract has the extraordinary ability to transport and eliminate fibers rapidly, the relation between transport and retention of asbestos fibers in the development of GI cancer is a relevant issue that has not been thoroughly investigated (12). According to the reviewed publications, exposure to asbestos has been mainly related with stomach cancer (13-17), esophageal cancer (18), and colon cancer (13, 19). However, there is still no significant evidence to prove this causal relationship (20). Table 1 evidences the association between asbestos and esophageal and small intestine cancer (21).
- Esophageal cancer
The relationship between the occupational exposure to asbestos and the development of esophageal cancer is still very controversial since it is a less frequent type of cancer. Esophageal cancer has many risk factors that are commonly found among the general population, such as smoking, alcohol consumption and esophageal reflux. Failure to consider these factors can undermine the validity of the conclusions drawn from different studies, as is the case with some of them (22).
If such relationship existed, whether it is dose-dependent or not is another aspect that causes uncertainty in this field. To test this, the most recent study that has been carried out proposed the division of the subjects under study into four different groups, depending on their degree of occupational exposure to asbestos. This study concluded that it was indeed a dose-dependent relation (23).
Considering this information, current evidence points to the positive association between asbestos exposure and the subsequent development of esophageal cancer. However, in most cases the statistical evidence is not strong enough to draw definitive conclusions (22, 23).
Furthermore, the results are not conclusive either in terms of the subtype of esophageal cancer that is most involved in this aspect. Thus, there are studies that have only found evidence of the studied relationship with adenocarcinoma, but not with the squamous cell carcinoma (which is the most common subtype) (24). Other studies do not have enough data to shed light on this matter (22, 23).
For these reasons, the studies carried out so far point to the need to continue doing research in this field so as to draw firm conclusions on the existence of this relationship.
- Stomach cancer
The relationship between asbestos exposure and stomach cancer has been studied with no conclusive results due to the low number of cases. A meta-analysis carried out in 2015 (25) determined through a systematic review the incidence and mortality rate of this type of cancer among workers exposed to asbestos.
Human cohorts were used for the studies that were taken into consideration. In these cohorts there was clear evidence of exposure to asbestos, mainly due to its use in cement production, shipyards, mining and textile industries. Furthermore, they showed a standardized incidence or mortality rate (as a surrogate of incidence, because of the relatively short survival time). The following studies were excluded: studies conducted on animals, studies with duplicate data, and studies in which not only exposure to asbestos was analyzed. From each selected cohort the following information was extracted: size, asbestos type, employment period, follow-up period, number of cancers detected, and randomization method. From 32 independent studies, 40 cohorts were collected. It was observed that 5 of those studies were about the incidence of stomach cancer (new diagnosed cases) while the rest focused on mortality. Most studies were carried out in Europe, 5 in Asia, 3 in America and 4 in Oceania. Finally, 13 studies had only taken male cohorts into consideration, while 5 considered only female cohorts.
The analysis of this work revealed a significantly higher risk of stomach cancer in the cohorts that were exposed only to crocidolite and mixed asbestos. Furthermore, the ratio had increased in Europe and Oceania. The main source of heterogeneity in the studies was the gender of the cohort and not the type of asbestos, geographical area, industry, sample size, or type of outcome.
The review considered concludes that workers exposed to asbestos are 1.19 times more likely to suffer stomach cancer than the general population. However, in this discussion two remarkable aspects are underlined:
The risk is higher in men, as other risk factors related to lifestyle such as alcohol and smoking are more frequent than in women (26). Studies indicate that smoking plays the most harmful and determining role in the development of stomach cancer, whereas alcohol promotes its progression.
Most cohorts encompassed miners and, consequently, bias should be considered, as some studies (27) indicate a higher risk of cancer in miners and millers. Finally, there is also evidence that relates it to coal mine dust.
- Colorectal cancer
As exposure to asbestos can cause gastric cancer, it is natural to consider that an association between asbestos and cancer in the most distal parts of the GI tract may exist. Thus, the evidence in the scientific literature of the relationship between asbestos exposure and colorectal cancer (CRC) is presented below.
Since 1980, experimental studies have shown an association between high-level ingestion of asbestos and the development of CCR in mice (29). Ingestion is a route of exposure to asbestos in humans, although not at such a high concentration as in this experiment. Consequently, this evidence is not strong enough and a comprehensive study in humans is needed.
Numerous cohort studies analyze this association, considering factors such as type and duration of exposure. A study conducted in Normandy, France, found a significant increase in the number of expected cases of CRC in male plant workers with a prolonged exposure of over 25 years (30).
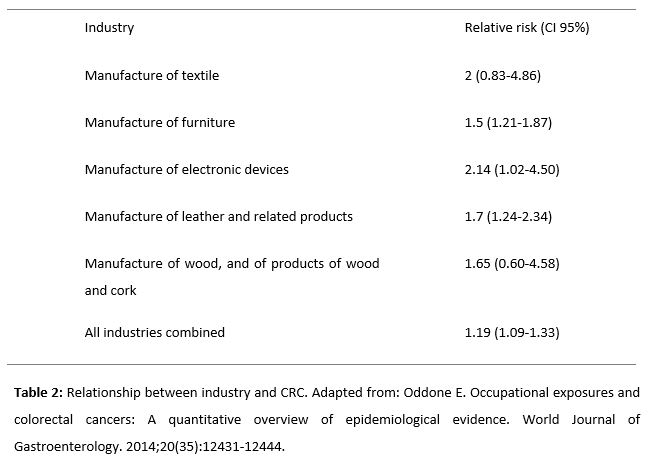
However, even when dealing with occupational exposure, the type of industry is significant to assess the increase in risk. Based on the numerous cohort studies of the scientific literature, Table 2 shows that the textile industry and the manufacture of electronic devices are the most prone to increase the risk of developing CRC (31).
There is also a slightly higher risk of developing CRC as a result of residential exposure to asbestos insulators, but no significant association has been established (32). Consequently, further studies are needed to determine whether such association exists, as it has been demonstrated with other types of cancer such as mesothelioma or lung cancer (33, 34).
In conclusion, there is evidence of the association between asbestos and CRC, although this is not as strong it is not as evident as in other type of cancers. Therefore, more comprehensive studies are required. In addition, the type of exposure should be considered, since differences between occupational and residential exposure have been observed.
- Conclusion
Asbestos is a mineral whose carcinogenic properties are well-known for their effects on the respiratory system. However, there is not enough evidence to support its association with tumors in other parts of the body, including the GI tract.
There is limited evidence of the association between asbestos exposure and esophageal tumors. Consequently, studies that consider confounding factors such as other known carcinogens are needed.
There is also evidence of an association between stomach cancer and occupational exposure to asbestos. Nevertheless, this evidence is not significant.
Different kinds of asbestos exposure, whether occupational or residential (in drinking water or as insulation material) have been associated with the development of CRC. However, like with other tumors, more cases are needed in order to establish a significant relationship.
Although there is a patent association between asbestos and GI cancer, more experimental and observational evidence is required. Nevertheless, it is difficult to collect observational evidence since asbestos is no longer in use.
It is necessary to confirm the carcinogenic effect of asbestos in the GI tract since asbestos levels in drinking water are not as controlled as they should be.
Conflicts of interest statement
The authors declare that there are no conflicts of interest in this article.
References
- Kim SJ, Williams D, Cheresh P, Kamp DW. Asbestos-Induced Gastrointestinal Cancer: An Update. J Gastrointest Dig Syst. 2013 Oct;3(3). pii: 135. Epub 2013 Sep 10. doi:10.4172/2161-069X.1000135
- Kjaerheim K, Ulvestad B, Martinsen JI, Andersen A. Cancer of the Gastrointestinal Tract and Exposure to Asbestos in Drinking Water among Lighthouse Keepers (Norway). Cancer Causes Control. 2005; 16:593–598. doi: 10.1007/s10552-004-7844-1
- Ramazzini C. Asbestos is Still with Us: Repeat Call for a Universal Ban. Am J Ind Med. 2011; 54:168–173.doi: 10.1002/ajim.20892
- US Department of Health and Human Services. Toxological Profile for Asbestos. Agency Toxic Subst Dis Regist. 2001;(September):327.
- IARC. Arsenic, Metals, Fibres, and Dusts. IARC Monogr Eval Carcinog Risks Hum. 2012; 100(PtC):11–465.
- WHO. Guidelines for Drinking-water Quality 4th ed., WHO, Geneva, p. 340. World Heal Organ. 2011;
- Kanarek MS. Epidemiological Studies on Ingested Mineral Fibres: Gastric and Other Cancers. IARC Sci Publ. 1989;90:428–437. PMID: 2744839
- Haque AK, Ali I, Vrazel DM et al. Chrysotile Asbestos Fibers Detected in The Newborn Pups Following Gavage Feeding of Pregnant Mice. J Toxicol Environ Health A. 2001;62(1):23–31. PMID: 11205533
- Hesdorffer ME, Chabot J, DeRosa C, Taub R. Peritoneal Mesothelioma. Curr Treat Options Oncol. 2008;9:180–190. doi: 10.1007/s11864-008-0072-2
- McConnell EE, Shefner AM, Rust JH, Moore JA. Chronic Effects of Dietary Exposure to Amosite and Chrysotile Asbestos in Syrian Golden Hamsters. Environ Health Perspect. 1983;53:11–25. doi: 10.1289/ehp.835311
- Kohyama N, Suzuki Y. Analysis of Asbestos Fibers In Lung Parenchyma, Pleural Plaques, and Mesothelioma Tissues of North American Insulation Workers. Ann NY Acad Sci. 1991;643:27–52. doi: 10.1111/j.1749-6632.1991.tb24442.x
- Bunderson-Schelvan M, Pfau JC, Crouch R, Holian A. Nonpulmonary Outcomes of Asbestos Exposure. J Toxicol Environ Health B Crit Rev. 2011; 14:122–152. doi: 10.1080/10937404.2011.556048
- Kinugawa K, Ueki A, Yamaguchi M et al. Activation of Human CD4+CD45RA+T Cells by Chrysotile Asbestos in Vitro. Cancer Lett. 1992;66:99–106. doi: 10.1016/0304-3835(92)90221-G
- Kanarek MS, Conforti PM, Jackson LA, Cooper RC, Murchio JC. Asbestos in Drinking Water and Cancer Incidence in the San Francisco Bay Area. Am J Epidemiol. 1980;112:54–72. doi: 10.1016/0021-9681(81)90065-5
- Andersen A, Glattre E, Johansen BV. Incidence of Cancer among Lighthouse Keepers Exposed to Asbestos in Drinking Water. Am J Epidemiol. 1993;138:682–687. PMID: 8237983
- Pira E, Pelucchi C, Piolatto PG, Negri E, Bilei T, La Vecchia C. Mortality from Cancer and Other Causes in the Balangero Cohort of Chrysotile Asbestos Miners. Occup Environ. Med. 2009;66:805–809. doi: 10.1136/oem.2008.044693.
- Hillerdal G. Gastrointestinal Carcinoma and occurrence of pleural plaques on pulmonary x-ray. J Occup Med. 1980;22:806–809. PMID: 7218058
- Kang SK, Burnett CA, Freund E, Walker J, Lalich N, Sestito J. Gastrointestinal cancer mortality of workers in occupations with high asbestos exposures. Am J Ind Med. PMID: 9131226
- Germani D, Belli S, Bruno C et al. Cohort mortality study of women compensated for asbestosis in Italy. Am J Ind Med. 1999;36:129–134. PMID: 10361597
- Institute of Medicine (US) Committee on Asbestos. Asbestos: Selected Cancers. Washington, USA: National Academies Press (US); 2006. doi: 10.17226/11665
- Bunderson-Schelvan M, Pfau JC, Crouch R, Holian A. Nonpulmonary outcomes of asbestos exposure. J of Toxicol Environ Health B Crit Rev. 2011;14(1–4):122–52. doi: 10.1080/10937404.2011.556048.
- Wu WT, Lin YJ, Li CY, et al. Cancer attributable to asbestos exposure in shipbreaking workers: A matched-cohort study. PLoS One. 2015;10(7):1–12. doi:10.1371/journal.pone.0133128.
- Clin B, Thaon I, Boulanger M et al. Cancer of the esophagus and asbestos exposure. Am J Ind Med. 2017;60(11):968–75. doi:10.1002/ajim.22769.
- Vermeulen R, Goldbohm RA, Peters S et al. Occupational asbestos exposure and risk of esophageal, gastric and colorectal cancer in the prospective Netherlands Cohort Study. Int J Cancer. 2014;135(8):1970–7. doi: 10.1002/ijc.28817.
- Peng W, Jia X, Wei B, Yang L, Yu Y, Zhang L. Stomach cancer mortality among workers exposed to asbestos: a meta-analysis. Journal of Cancer Research and Clinical Oncology. 2014;141(7):1141-1149. doi: 10.1007/s00432-014-1791-3
- Li L, Ying XJ, Sun TT et al. Overview of methodological quality of systematic reviews about gastric cancer risk and protective factors. Asian Pac J Cancer Prev. 2012;13(5):2069-2079. doi: 10.7314/APJCP.2012.13.5.2069
- Musk AW, de Klerk NH, Reid A et al. Mortality of former crocidolite (blue asbestos) miners and millers at Wittenoom, Occup Environ Med. 2008;65(8):541-543. doi: 10.1136/oem.2007.034280
- Ames RG. Gastric cancer and coal mine dust exposure: a case-control study. Cancer. 1983;52: 1346-1350. PMID: 6883295
- Donham K, Berg J, Will L, Leininger J. The effects of long-term ingestion of asbestos on the colon of F344 rats. Cancer. 1980;45(S5):1073-1084. PMID: 6244076
- Boulanger M, Morlais F, Bouvier V et al. Digestive cancers and occupational asbestos exposure: incidence study in a cohort of asbestos plant workers. Occupational and Environmental Medicine. 2015;72(11):792-797. doi: 10.1136/oemed-2015-102871
- Oddone E. Occupational exposures and colorectal cancers: A quantitative overview of epidemiological evidence. World Journal of Gastroenterology. 2014;20(35):12431-12444. doi: 10.3748/wjg.v20.i35.12431.
- Korda R, Clements M, Armstrong B et al. Risk of cancer associated with residential exposure to asbestos insulation: a whole-population cohort study. The Lancet Public Health. 2017;2(11):e522-e528. doi: 10.1016/S2468-2667(17)30192-5
- Goswami E, Craven V, Dahlstrom D, Alexander D, Mowat F. Domestic Asbestos Exposure: A Review of Epidemiologic and Exposure Data. International Journal of Environmental Research and Public Health. 2013;10(11):5629-5670. doi: 10.3390/ijerph10115629.
- Lacourt A, Gramond C, Rolland P et al. Occupational and non-occupational attributable risk of asbestos exposure for malignant pleural mesothelioma. Thorax. 2014;69(6):532-539. doi: 10.1136/thoraxjnl-2013-203744