Regina Gálvez-López ¹ , Marta Rodríguez-Camacho ¹ , Andrés Soriano-Mateos ¹ , Eloi Querol-Carranza ¹
1 Estudiante del Máster en Neurociencias Básicas Aplicadas y Dolor de la Universidad de Granada (UGR)
TRANSLATED BY:
Julia Cervilla-Carrión ² , Andrea Martín-Manzano ² , María Luisa Martín-Vázquez ² , Cristina de-la-Torre-Sánchez ² , Carolina Sobrino-Cano ² , Rocío Soto-García ²
2 Student of the BA in Translation and Interpreting at the University of Granada (UGR)
Neuropathic pain (NP) is a difficult-to-manage kind of pain that affects 10% of the population. Nowadays, there are some agents that can be used for its treatment, and they are grouped according to different lines. However, their pharmacological effectiveness is only proved on a reduced number of patients. For this reason, new therapeutic strategies need to be developed. Certain molecules currently are on the initial stages of human clinical trials, and many of them are showing encouraging results. Furthermore, there is an increasingly accurate knowledge about the different molecular pathways related to NP. This advance is promoting the development of new molecules with potential therapeutic targets. Some of these agents are being tested with animal models, and they are showing a significant analgesic potency. Finally, non-pharmacological treatment in NP is becoming more important in clinical practice through alternatives such as radio frequency, stimulation, and nerve block therapy. The aim of this work is to review the state of art of NP treatment. For that purpose, the agents approved until today and their main action mechanisms should be considered, with a special emphasis on some of the latest molecules used on human clinical
trials and those which are in development with animal models. Moreover, some of the main non-pharmacological strategies used to manage pain nowadays are also reviewed.
Keywords: neuropathic pain, new agents, pharmacological treatment, non-pharmacological treatment, clinical trial.
- Introduction
Neuropathic Pain (NP) is caused by a disease or a direct injury of the central or peripheral nervous system, and it comprises a wide range of etiological causes (1, 2). Although there are some difficulties to determine the prevalence of NP, it is estimated that 7-10% of the population suffer from it. Accordingly, women and people aged over 50 are the most common population groups affected by NP (2). Normally, the most frequently affected areas are the hind neck, the lumbar region, and lower and higher extremities (3). Among the different pathologies that are commonly related to this kind of pain, it is important to highlight peripheral neuropathies, post-herpetic neuralgia, traumatic nerve injury, spinal cord injury, multiple sclerosis, stroke, and various types of cancer (3).
NP is considered by many experts as one of the painful syndromes that are most difficult to manage (2). The treatment complexity of this syndrome has led to the development of multiple clinical trials, meta-analyses, and clinical guidelines such as those of the International Association for the Study of Pain (IASP) (4). According to the scientific evidence and these guidelines, the pharmacological treatment of NP usually follows different lines, each of them with its own pharmacological group (5). However, in a significant number of patients, the relief obtained is quite limited after applying all of the pharmacological lines.
Currently, there are some agents for NP treatment on their first stages of clinical trials that are achieving promising results (6, 7). On the other hand, several potential therapeutic targets have been proposed based on a better understanding of NP physiopathology and on the results achieved with animal models (8). Finally, it is important to bear in mind that NP treatment and management include non-pharmacological measures, and that there are some therapies, such as stimulation and nerve blocks, that are increasingly becoming relevant (9).
This article aims at reviewing the state of art regarding NP pharmacological treatment by examining the latest international clinical guidelines, the treatments with better results in later stage clinical trials, and the experimental development of agents in animal models. Furthermore, some of the potential therapeutic targets that have recently been proposed are also reviewed.
- Neuropathic pain pharmacological treatment
With regard to the NP pharmacological treatment, several treatment lines have demonstrated their efficacy, highlighting anticonvulsants, tricyclic antidepressants (TCAs), and selective serotonin and norepinephrine reuptake inhibitors (SNRIs).
- First-line agents
TCAs (e.g. amitriptyline) belong to this group. They inhibit serotonin and norepinephrine reuptake, which increases the top-down inhibitory control of pain (10). However, they also act on the sodium channel, beta-2 adrenergic receptors, and N-methyl-d-aspartate (NMDA) receptors, thus producing side effects such as sedation, dizziness, dry mouth, and orthostatic hypotension (5). Furthermore, SNRIs (e.g. duloxetine and venlafaxine) are also considered first-line agents, and have had positive results in many cases, being nausea their most common side effect (11). Finally, anticonvulsants (e.g. pregabalin and gabapentin) have also demonstrated their efficacy in NP pharmacological treatment. They reduce the calcium entry in the dorsal horn of the spinal cord, decreasing the central sensitization (10). As they often cause drowsiness, dizziness, and edemas, it is recommended to start the treatment at low doses (5, 11).
- Second-line agents
Tramadol can be mentioned among the second-line agents. It is a weak opioid which acts as a weak mu receptor agonist, and as an SNRI. It is recommended to use Tramadol with caution due to the dependence and abuse risks, and also due to its side effects, including nausea, confusion, drowsiness and the reduction of seizure thresholds (5, 11, 12).
Other second-line treatments, especially recommended for peripheral NP, are topical agents like lidocaine and capsaicin. 5% lidocaine transdermal patches block the sodium channel and decrease nerve depolarization. 0.075% capsaicin cream and 8% capsaicin patches cause the desensitization of the transient receptor potential vanilloid 1, and they modulate pain signaling (1, 5).
- Third-line agents
Strong opioids (e.g. oxycodone and morphine) have recently been promoted to third-line agents, as opposed to their prior consideration as first or second-line treatment (12). This is primarily due to their potential side effects, risks and the need for them to be monitored in NP treatment. These opioids are more likely than tramadol to cause withdrawal, dependence, and abuse. Moreover, opioids can induce nausea, constipation, drowsiness, respiratory depression; and, eventually, hyperalgesia, and endocrine disruptions (5, 11).
- Fourth-line agents
Although some agents are probably effective in certain subgroups of patients, the current recommendations for the use of all other drug treatments for NP are weak or inconclusive (1). Recommended fourth-line treatments include other anticonvulsants (e.g. oxcarbazepine, topiramate), other strong opioids (tapentadol), and botulinum toxin, among others (11, 12).
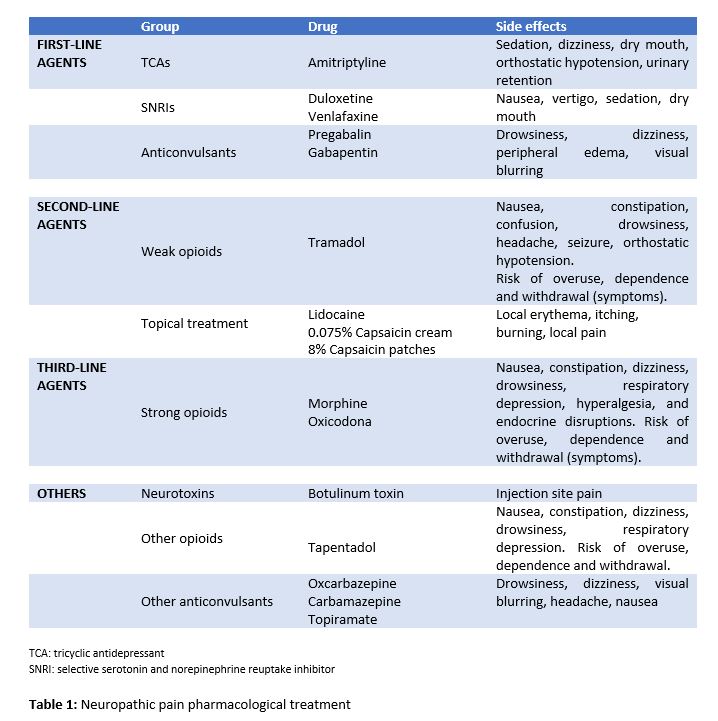
Despite the availability of a large number of therapies, drugs largely used to relieve NP usually have a limited efficacy or dose-limiting side effects (Table 1). Therefore, the validation of novel agents and the study of potential therapeutic targets is becoming increasingly necessary (13).
- New molecules in clinical trials
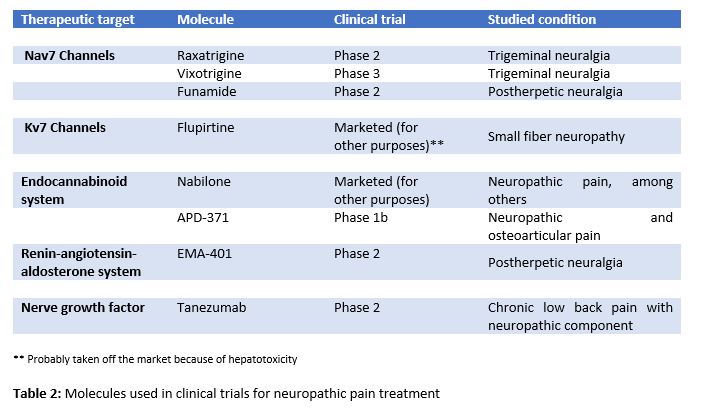
In the last few years, some clinical trials with active principles, which were targeted to different molecules to relieve NP, have been conducted in humans (6, 7). Some of the drugs currently under development are focused on voltage-gated ion channels (Na+, K+), on the endocannabinoid system, on the renin-angiotensin-aldosterone system (RAAS) or on the nerve growth factor (NGF) receptor (Table 2).
- Voltage-gated ion channels
- 7 sodium channels
Nav1.7 is the voltage-gated sodium channel that predominantly expresses in nociceptors in the dorsal root of the spinal cord. It contributes to the initiation and the upstroke phase of the peripheral nociceptor action potential, as well as to synaptic transmission and to neuropeptide release in the dorsal horn of the spinal cord (6). Raxatrigine (14), vixotrigine (15), and funamide (16) are highly selective inhibitors of Nav1.7 in phase 2 trial (raxatrigine, funamide) and phase 3 trial (vixotrigine) to treat NP in trigeminal neuralgia in the case of raxatrigine and vixotrigine, and postherpetic neuralgia in the case of funamide.
- Kv7 potassium channels
Kv7 is a voltage-gated non-inactivating potassium channel, and its down-regulation has been implicated in several hyperexcitability-related disorders. Flupirtine, a Kv7 channel activator, has recently showed analgesic effects on refractory NP treatment due to small fiber neuropathy. However, hepatotoxicity has been reported with flupirtine (7). Kv7.5, the main Kv7 subunit which is expressed by C-fibers, focuses on the possible role of selective Kv7.5 enhancers in NP treatment (6, 7).
- Endocannabinoid system
The endocannabinoid system includes two receptors (CB1 and CB2), their endogenously produced ligands (anandamide and 2-arachidonoylglycerol), and a wide metabolic machinery. Nabilone, a synthetic cannabinoid approved for nausea and vomiting associated with chemotherapy, has showed efficacy in the treatment of different types of pain, including NP (7, 11). Notably, APD371, a highly selective agonist of the CB2, has obtained positive results in a phase Ib clinical trial in NP and osteoarthritic treatment (7). However, the development of selective agonists of the cannabinoid receptors is complex because they are involved in diverse physiological processes. Some CB2 ligands produce no major side effects at the central nervous system.
- Renin-angiotensin-aldosterone system
The union of angiotensin II (Ang II) to its receptors, AT1 and AT2, acts as a neuromodulator in the brain and in the spinal cord. Ang II participates in the central and peripheral regulation of sensory nervous information, nociception, and taste and visual system. Furthermore, the expression of AT1 receptors and the conversion of Ang II to Ang III in central nervous system neurons are involved in a descending pain modulation (6, 13). EMA401 is a selective antagonist of AT2 receptors that has proved to be an effective option in NP treatment in patients with postherpetic neuralgia in phase II of clinical trials (17).
- Nerve growth factor
This growth factor is involved in the painful physiological response to harmful stimuli, and it is increased in a great variety of cases of acute and chronic pain. For this reason, molecules that act as NGF antagonists have been developed (6,13). Tanezumab, a humanized IgG2 that blocks the interaction between NGF and its TrkA and p75 receptors, has demonstrated a better analgesic efficacy in patients with neuropathic component in chronic back low pain than naproxen and placebo in phase II clinical trials (6).
- New molecular targets
Nowadays, over one hundred molecules with a therapeutic potential in NP treatment are being developed. Most of the studies have been conducted on animals. Although many results are positive, they are not directly applicable to humans (13, 18). Some molecules of special interest are sigma receptors, ephrins, and endoplasmic reticulum stress (ERS) receptors.
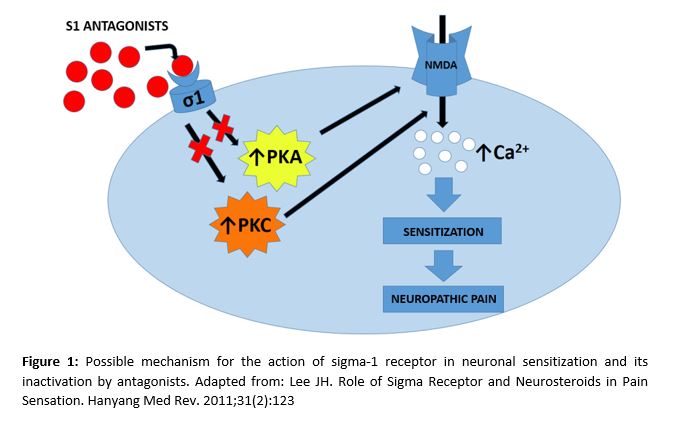
- Sigma receptors
Sigma receptors are chaperon-like proteins that act in combination with NMDA channels and enable calcium influx in neurons. The sigma-1 receptor plays an essential role in neuronal sensitization and in chronic pain development. This is why its action mechanism (Figure 1) is being thoroughly studied (19-21). Moreover, it has been demonstrated that nerve injuries increase the number of sigma-1 receptors in the damaged neurons (8). Some studies conducted on NP-induced mice with paclitaxel show that both pharmacological blockade with sigma-1 antagonists, as well as the genetic deactivation of it in knockout mice, inhibit the NP caused by paclitaxel (21).
- Ephrins
Ephrins are receptor tyrosine kinase ligands involved in neural development. They present receptors in the laminae I-III of the dorsal horn and in dorsal root ganglion (DRG) neurons (22). In addition, they regulate the NMDA-dependent synaptic activity and they participate in spinal pain processing. Ephrins participate as well in upstream pain mechanisms through phosphatidylinositol 3-kinase (23) and protein kinase C-γ (24), increasing the excitability of nociceptive neurons and their synaptic plasticity.
4.3 Endoplasmic reticulum stress receptors
In the presence of stress molecules, an incorrect protein folding occurs in the endoplasmic reticulum (ER). As a defense mechanism, the unfolded protein response (UPR) mediated by chaperones is triggered. This ER response can also be activated by pro-inflammatory agents. Binding immunoglobulin proteins (BiP) belong to the chaperone family. Studies conducted in murine models with orofacial inflammatory pain showed an increase in BiP (25), suggesting that chronic activation of the UPR system may induce neuronal vulnerability in response to NP stimuli.
4.4 β-catenin, Wnt, Ryk
They are molecules involved in neuronal metabolism and development. It has been observed an increase of Wnt3 in the dorsal horn of rats whose sciatic nerve had been ligated. A Wnt/β-catenin signaling inhibitor, XAV939, attenuated NP sensitivity (26). Blocking Ryk in mice suppresses neuronal hyperexcitability and neuroplasticity in the dorsal horn. Other studies suggest that intervening on these receptors may have a therapeutic potential in NP treatment (18, 27, 28).
4.5 D-amino acid oxidase
It is a peroxisomal enzyme that catalyzes the oxidative deamination of D-amino acids. Intrathecal administration of D-amino acids oxidase (DAAO) inhibitors in murine models revealed NP reduction (29) and formalin-induced tonic phase pain (30).
4.6 Epigenetic modifications
The epigenetic modification of genes related to the expression of receptors, ion channels and other mediators altered in NP could be a therapeutic way. Several studies suggest that there is an alteration in the expression of these mediators by methyltransferases, demethylases, histone acetyltransferases (HATs) and histone deacetylases (HDACs). In this line, the influence of HATs on the expression of chemokines and that of HDACs on the expression of cytokines in glial cells and macrophages have been demonstrated (31).
- Neuropathic pain non-pharmacological treatment
There is a great variety of non-pharmacological techniques that may complement NP treatment by playing an important role in the psychological well-being of the patient, as well as in the evolutionary course of pain (9).
5.1. Non-invasive therapies
Several clinical trials in humans have studied the relationship between physical activity and pain sensitivity. Physical exercise is associated with a greater tolerance to pain in chronic low back pain, fibromyalgia, osteoarthritis, and peripheral NP (32, 33). Physiotherapy and techniques such as mirror therapy and graded motor imagery also appear to be beneficial in NP (1, 9). On the other hand, psychotherapy, especially cognitive-behavioral therapy, has been used to promote the patient’s active participation in their painful condition and to reduce its consequences at affective, functional and social levels, although there is no evidence to prove its efficacy in NP (1).
5.2. Invasive and minimally invasive therapies
The invasive techniques used in pain treatment seem to be a valid therapeutic alternative for those patients with NP for whom other treatments have not been effective (1). The importance of these techniques has grown in the last few years, and it is expected to keep growing. They include radio frequency (RF), neurostimulation, and nerve block therapy, among others.
5.2.1. Radio frequency
RF is a minimally invasive technique that produces electromagnetic and thermal fields in order to regulate the channel expressions in DRG. It also contributes to the neuromodulation of the nervous system (34, 35). Pulsed radiofrequency (PRF) is used to treat joint and muscle pain, and it has been proved to be effective in cases of radicular pain and neuralgia (36).
5.2.2. Neurostimulation
Spinal cord stimulation (SCS) and transcutaneous electrical nerve stimulation (TENS) are two remarkable techniques used in neurostimulation. On the one hand, SCS acts at the back of the spinal cord and modulates the stimuli transmitted by the C-fibers through Aβ-fibers (1). However, it is still unknown how this produces analgesia (9). SCS is effective in the treatment of syndromes like failed back surgery syndrome and complex regional pain syndrome, among others (37). On the other hand, TENS is a widely used non-invasive technique that activates the descending inhibitory systems, although its efficacy has not been proved by any research yet (38).
5.2.3. Nerve block therapy
Nerve block therapy is a widely used procedure for chronic pain conditions. It is useful for both NP diagnosis and treatment (39). Guirguis et al. (40) reported the case of a patient with postoperative residual abdominal pain, which was refractory to invasive techniques. To determine the origin of the pain, it was necessary to perform a nerve block in the area where the patient had the pain. Then, the peripheral source of the abdominal pain was confirmed and, by inserting a catheter which continuously infused a local anesthetic, the analgesic effect was maintained.
- Conclusions
NP treatment is a complex task that can be approached by both pharmacological and non-pharmacological methods. There are several groups inside the pharmacological ones. First-line agents include TCA, SNRI and anticonvulsive agents such as pregabalin and gabapentin. Second-line agents include weak opioids (tramadol) and topical lidocaine or capsaicin. Among third-line agents, strong opioids like morphine stand out. Finally, fourth-line agents are represented by the use of strong opioids, botulinum toxin, and other anticonvulsive agents. Although there is a wide variety of agents available, their overall efficacy is limited just to certain patients. Therefore, more powerful molecules should be designed.
Nowadays, some of the most important molecules in clinical trials act on ion channels (Nav 1.7, Kv 7), on the endocannabinoid system, on the RAAS or on NGF receptors. Moreover, some other molecules are showing interesting results in animal models. The most relevant ones are those which interact with sigma receptors, ERS receptors, the β-catenin/Wnt/Ryk triad, DAAO, and several epigenetic regulators. To conclude, non-pharmacological pain treatment is becoming increasingly important. This kind of treatment can be applied by using non-invasive therapies (physical activity, physiotherapy, graded motor imagery, psychotherapy), and invasive or minimally invasive therapies (RF, neurostimulation, and nerve block therapy).
Conflicts of interest statement
The authors declare that there are no conflicts of interest in this article.
References
- Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic Pain. Nat Rev Dis Prim. 2017;3:1–45.
- Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008 Jun;136(3):380–7.
- Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain [Internet]. 2014 Apr [cited 2019 Mar 24];155(4):654–62.
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015 Feb;14(2):162–73.
- Galvez R. Guía de Práctica Clínica sobre el Tratamiento Farmacológico del Dolor Neuropático Periférico en Atención Primaria. 2016.
- Sałat K, Kowalczyk P, Gryzło B, Jakubowska A, Kulig K. New investigational drugs for the treatment of neuropathic pain. Expert Opin Investig Drugs. 2014;23(8):1093–104.
- Yan Y yi, Li C yuan, Zhou L, Ao L yao, Fang W rong, Li Y man. Research progress of mechanisms and drug therapy for neuropathic pain. Life Sci. 2017;190:68–77.
- Bangaru ML, Weihrauch D, Tang Q-B, Zoga V, Hogan Q, Wu H. Sigma-1 receptor expression in sensory neurons and the effect of painful peripheral nerve injury. Mol Pain. 2013 Sep;9:47.
- Xu L, Zhang Y, Huang Y. Translational Research in Pain and Itch. Vol. 904. 2016. p. 117-130.
- Attal N. Pharmacological treatments of neuropathic pain: The latest recommendations. Rev Neurol (Paris). 2019;175(1–2):46–50.
- Mu A, Weinberg E, Clarke H. Pharmacologic management of chronic neuropathic pain. Can Fam Physician. 2017;63:844–52.
- Finnerup NB, Attal N, Haroutounian S, Moore A, Raja SN, Rice ASC. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2016;14(2):162–73.
- Bouhassira D, Attal N. Emerging therapies for neuropathic pain: new molecules or new indications for old treatments? Pain. 2018;159(3):576–82.
- Zheng Y, Wang W, Li Y, Yu Y, Gao Z. Enhancing inactivation rather than reducing activation of Nav1.7 channels by a clinically effective analgesic CNV1014802. Acta Pharmacol Sin. 2018;39(4):587–96.
- Di Stefano G, Truini A, Cruccu G. Current and Innovative Pharmacological Options to Treat Typical and Atypical Trigeminal Neuralgia. Drugs. 2018;78(14):1433–42.
- Price N, Namdari R, Neville J, Proctor KJW, Kaber S, Vest J, et al. Safety and Efficacy of a Topical Sodium Channel Inhibitor (TV-45070) in Patients With Postherpetic Neuralgia (PHN). Clin J Pain. 2017 Apr;33(4):310–8.
- Rice ASC, Dworkin RH, McCarthy TD, Anand P, Bountra C, McCloud PI, et al. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet (London, England). 2014 May;383(9929):1637–47.
- Khangura RK, Sharma J, Bali A, Singh N, Jaggi AS. An integrated review on new targets in the treatment of neuropathic pain. Korean J Physiol Pharmacol. 2019 Jan;23(1):1–20.
- Merlos M, Romero L, Zamanillo D, Plata-Salamán C, Vela JM. Sigma-1 Receptor and Pain. In: Handbook of experimental pharmacology. 2017. p. 131–61.
- Lee J-H. Role of Sigma Receptor and Neurosteroids in Pain Sensation. Hanyang Med Rev. 2011 ;31(2):123.
- Nieto FR, Cendán CM, Sánchez-Fernández C, Cobos EJ, Entrena JM, Tejada MA, et al. Role of Sigma-1 Receptors in Paclitaxel-Induced Neuropathic Pain in Mice. J Pain. 2012 Nov;13(11):1107–21.
- Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003 Aug;23(21):7789–800.
- Yu L-N, Zhou X-L, Yu J, Huang H, Jiang L-S, Zhang F-J, et al. PI3K Contributed to Modulation of Spinal Nociceptive Information Related to ephrinBs/EphBs. Baccei ML, editor. PLoS One. 2012 Aug;7(8):e40930.
- Zhou X-L, Zhang C-J, Wang Y, Wang M, Sun L-H, Yu L-N, et al. EphrinB–EphB signaling regulates spinal pain processing via PKCγ. Neuroscience. 2015 Oct;307:64–72.
- Yang F, Whang J, Derry WT, Vardeh D, Scholz J. Analgesic treatment with pregabalin does not prevent persistent pain after peripheral nerve injury in the rat. Pain. 2014 Feb;155(2):356–66.
- Itokazu T, Hayano Y, Takahashi R, Yamashita T. Involvement of Wnt/β-catenin signaling in the development of neuropathic pain. Neurosci Res. 2014 Feb;79:34–40.
- Yang QO, Yang W-J, Li J, Liu F-T, Yuan H, Ou Yang Y-P. Ryk receptors on unmyelinated nerve fibers mediate excitatory synaptic transmission and CCL2 release during neuropathic pain induced by peripheral nerve injury. Mol Pain. 2017 Jan 31;13:174480691770937.
- Gao K, Wang Y, Yuan Y, Wan Z, Yao T, Li H, et al. Neuroprotective effect of rapamycin on spinal cord injury via activation of the Wnt/β-catenin signaling pathway. Neural Regen Res. 2015 Jun;10(6):951.
- Zhao J, Yuan G, Cendan CM, Nassar MA, Lagerström MC, Kullander K, et al. Nociceptor-Expressed Ephrin-B2 Regulates Inflammatory and Neuropathic Pain. Mol Pain. 2010 Jan 29 ;6:1744-8069-6–77.
- Chen X-L, Li X-Y, Qian S-B, Wang Y-C, Zhang P-Z, Zhou X-J, et al. Down-regulation of spinal d-amino acid oxidase expression blocks formalin-induced tonic pain. Biochem Biophys Res Commun. 2012 May 11;421(3):501–7.
- Penas C, Navarro X. Epigenetic Modifications Associated to Neuroinflammation and Neuropathic Pain After Neural Trauma. Front Cell Neurosci. 2018 Jun 7;12:158.
- Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014 Apr 4;8:102.
- Kroll HR. Exercise Therapy for Chronic Pain. Phys Med Rehabil Clin N Am. 2015;26(2):263–81.
- Liu Y, Feng Y, Zhang T. Pulsed Radiofrequency Treatment Enhances Dorsal Root Ganglion Expression of Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels in a Rat Model of Neuropathic Pain. J Mol Neurosci. 2015 Sep 28;57(1):97–105.
- Abejón D, Parodi E, Blanco T, Cavero V, Pérez-Cajaraville J. Radiofrecuencia pulsada del ganglio dorsal de las raíces lumbares. Rev la Soc Española del Dolor. 2011;18(2):135–40.
- Chang MC. Efficacy of Pulsed Radiofrequency Stimulation in Patients with Peripheral Neuropathic Pain: A Narrative Review. Pain Physician. 2018;21(3):E225–34.
- Wong SSC, Chan CW, Cheung CW. Spinal cord stimulation for chronic non-cancer pain: A review of current evidence and practice. Hong Kong Med J. 2017;23(5):517–23.
- Vance CGT, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014 May;4(3):197–209.
- Wijayasinghe N, Andersen KG, Kehlet H. Neural Blockade for Persistent Pain After Breast Cancer Surgery. Reg Anesth Pain Med. 2014;39(4):272–8.
- Guirguis MN, Abd-Elsayed AA, Girgis G, Soliman LM. Ultrasound-Guided Transversus Abdominis Plane Catheter for Chronic Abdominal Pain. Pain Pract. 2013 Mar;13(3):235–8.