Tom Stoelers¹, Roy Nicolás Álvarez-Garrido ¹ , Raúl Navas-Rutete ¹ , Elena Rosado-Gutiérrez ¹ , Cristina De-Luis-Pareja ¹ , Natalia Astasio-García ¹
¹Estudiante del Grado en Medicina de la Universidad de Granada (UGR)
TRANSLATED BY:
Esperanza González-Moreno ² , Antonio Hermán-Carvajal ² , Miguel Sillero-Romero ² , Claudia Carrasco-Barrios ² , Elena Ruiz-López-Ocón ² , Celia Fernández-Gamarra ²
2 Student of the BA in Translation and Interpreting at the University of Granada (UGR)
Introduction: Schizophrenia is a psychiatric disorder that could be related to cannabis use.
Pathogenic relationship between cannabis and schizophrenia: Environmental and biological factors are involved. The physiopathology of schizophrenia is characterized by physiological and anatomic alterations of the central nervous system. Cannabis alters the central nervous system, the immune system, and other tissues. Factors involved in the relationship between cannabinoids and schizophrenia: The mutation in the gene that encodes neuregulin-1 could be related to a higher sensitivity to the sedative effects of cannabinoids and to the development of extreme anxiety induced by stress. Consequently, this could represent a potential risk factor. Another potential risk factor is the presence of adverse life events, such as experiencing a breakup during adolescence or even belonging to a dysfunctional family. Furthermore, having a lower socioeconomic status could be related to a higher frequency of drug abuse. Discussion: According to the evidence shown in some cohort studies, cannabis use increases the possibility of experiencing psychotic symptoms. In addition, it has been observed that those who suffer from non-psychotic psychiatric diseases are not appropriate for studies on cannabis studies and its relationship with a potential development of schizophrenia. A potential source of bias in these types of research studies is the use of non-anonymous and self-administered use questionnaires. Some confusing factors to consider when analyzing the potential relationship between schizophrenia and cannabis use are socioeconomic aspects, psychological aspects, sex, age and the ethnic group of the individuals. Conclusion: It seems that there is enough evidence of the causal relationship between cannabis and schizophrenia. However, it would be necessary to study how to avoid the development of this disorder in a sample of at least 1300 individuals at risk of suffering from schizophrenia induced by cannabis. It is possible to conclude from this review that there is a causal relationship between cannabis use and the development or worsening of schizophrenia.
Keywords: schizophrenia, cannabis, neuregulin-1, stress, risk factor, adolescence, adverse life events.
- Introduction
According to the DSM-5 (1), schizophrenia is a disorder in which the patient must have experienced at least two or more of the following symptoms, each present for a significant portion of time during a one-month period (or less if successfully treated): delusions, hallucinations, disorganized speech, grossly disorganized or catatonic behavior, and negative symptoms. At least one of the symptoms must be the presence of delusions, hallucinations, or disorganized speech. Schizophrenia is diagnosed when such symptoms persist for at least six months, during which the patient must experience at least a month of active symptoms (or less if successfully treated, as mentioned above), Furthermore, schizophrenia causes decreased levels of functioning regarding one or more life aspects, such as work or interpersonal relationships, among others. Nowadays, it is estimated that schizophrenia affects around 0.3 to 0.7% of the population at some point in their life. However, some variations related to different factors, like the ethnic group of the individual, have been found.,.
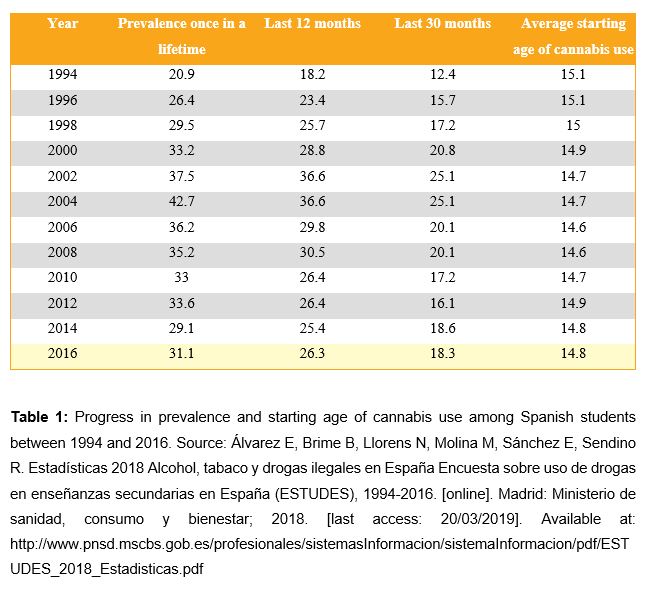
Cannabis is also reviewed in this article, since there is an increase of the possibility of suffering from a psychotic disorder when there is cannabis use. Throughout history, this drug has become increasingly popular, ranking today as the most used illegal drug in the European Union. In addition, it is estimated that 16% of the population aged 15 to 64 have used cannabis at least once in their lifetime (2). As shown in Table 1, there has been an increase of the prevalence of cannabis use among Spanish secondary education students from 1994 to 2004 (3), when it reaches a peak (indicated with the numbers in red in the figure). Right after that peak, cannabis use rate decreases. However, according to recent surveys, an increasing prevalence of cannabis use can be observed once again. Nevertheless, the starting age of cannabis use has remained constant through all the surveys.
Taking into account all this information, the main goal of this review is to observe the potential causal relationship between cannabis use and the subsequent diagnosis of schizophrenic disorder by reviewing the available bibliography.
- Pathogenic relationship between cannabis and schizophrenia
2.1 Schizophrenia pathogenesis
The pathogenesis of schizophrenia is still unclear. It is a complex disease which results from the interaction between genetic and environmental factors. This was determined after observing the concordance rate of schizophrenia in monozygotic twins, which is about 40-50%, whilst the concordance rate in dizygotic twins is only about 10-15% (4). Based on its symptoms and signs, these are the neurophysiological hypotheses about schizophrenia pathogenesis:
- Dopamine hypothesis: patients with schizophrenia have an alteration in dopamine release. This results in a dopaminergic hyperactivity in the corpus striatum, and a dopaminergic hypofunction in extrastriatal areas (5).
- Glutamate hypothesis: patients with schizophrenia have a hypofunction of the glutamate receptors (NMDA) (6).
- Interneuron dysfunction hypothesis: patients with schizophrenia have a reduced interneuron activity in the dorsolateral prefrontal cortex. Among the various types of interneurons, parvalbumin-expressing GABAergic interneurons are the ones affected. These interneurons inhibit pyramidal neurons (7).
Furthermore, certain morphological abnormalities have been found in patients with schizophrenia. For instance, brain volume and, specifically, hippocampal volume are reduced; whilst ventricular volume is increased (8).
2.2 Cannabis physiology
The main psychoactive substance in cannabis is delta-9-tetrahydrocannabinol (THC). This substance is an agonist of the cannabinoid receptor. The cannabinoid receptor is a G protein-coupled receptor which inhibits adenylcyclase and stimulates potassium conductance. There are two types of receptors:
- CB1: It is found in the central nervous system (CNS). It inhibits the release of acetylcholine, L-glutamate, GABA, noradrenalin, dopamine, and 5-hydroxytryptamine.
- CB2: It is found in immune system tissues, in peripheral nerve endings and in deferent ducts. It is also involved in the regulation of immune and inflammatory responses.
THC has several neuropsychiatric effects due to its union to CB1 in the CNS: it triggers a state of euphoria in the patient; it reduces anxiety, the sense of alert and tension; it slows reaction time; and it impairs attention, concentration, short term memory, and risk assessment (9). It also reduces cholinergic activity and blood flow in the hippocampus.
2.3 Relationship between cannabis and schizophrenia
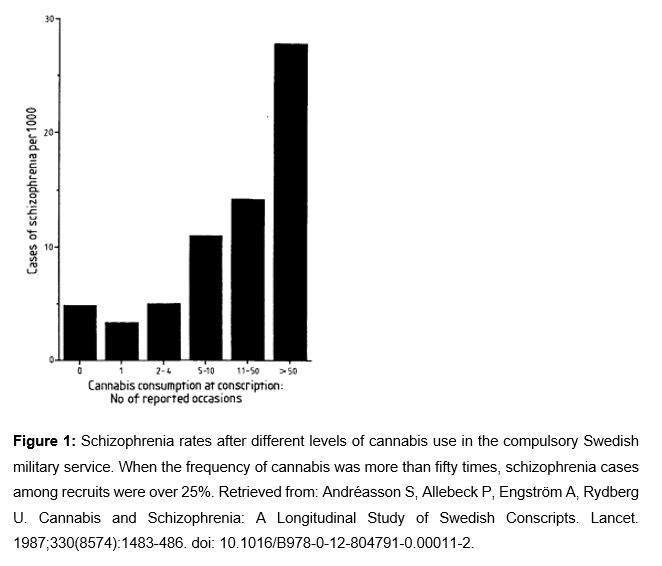
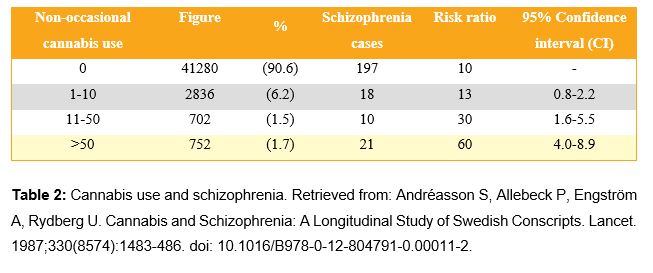
Several cases of psychosis have been observed after using high doses of cannabis (11). Moreover, some cases of patients with controlled schizophrenia whose psychosis was exacerbated after cannabis use have also been observed (12). A prospective study with 45,570 Swedish individuals found a dose-dependent relationship between the frequency of cannabis use and schizophrenia development risk in the following 15 years (10). As shown in Figure 1 and in Table 2, the increase of self-declared cannabis use is related to an increase of schizophrenia cases among the individuals of the study. In schizophrenia, dopamine, glutamate, and GABA activity are altered. THC alters the activity of neurotransmitters due to its agonist action in CB1. Furthermore, in schizophrenia, hippocampus volume and its blood flow are reduced. Taking all of this into consideration, it seems that there are some biological mechanisms related to the exposure to THC with schizophrenia.
- Factors affecting the relationship between cannabinoids and schizophrenia
3.1. Genetic factors
Neuregulin-1 (NRG1) type IV is a protein that affects the central nervous system and embryogenesis development. These isoforms participate in neuronal migration and differentiation. They affect glutamatergic synaptic vesicles, acting through NMDA receptors in the expression and the activation of glutamate and other receptors, such as gamma-aminobutyric acid (GABA) receptors.
The gene encoding NRG1 is located on chromosome 8p. NRG1 type IV has only been detected in the brain and its alteration was associated with a decrease in grey and white matter volume, with white matter structural defects and with increased ventricular volume. A study in mice showed that neuregulin-1 mutant mice were more sensitive to the sedative action of cannabinoids and that they developed stress-induced anxiety. Furthermore, in a genome-wide study of a sample of African-American individuals, NRG1 was found to be similar to a susceptibility gene for cannabis dependence. Studies on humans did not have uniform results regarding the relationship between a mutation in NRG1 and schizophrenia. However, collected data on the connection between mutations in NRG1 and lower stress tolerance and increased sensitivity to cannabinoids imply that it can serve as a risk factor. This is due to the fact that the subjects suffering from schizophrenia, as well as their relatives, experienced a higher number of adverse life events and a higher drug use (e.g. tobacco, alcohol, cannabis) (13).
3.2. Age and sex
Evidence suggests that there is a strong association between the cannabis use at an early age and the subsequent development of mental disorders (e.g. schizophrenia). On the contrary, such association was not observed in patients who began cannabis use at a later age (13).
Regarding gender differences, studies of cohorts of adolescents who had experienced stressful life events suggested that women participating in the study were more affected by stress than men. This finding might be confusing when observing the relationship between stressful life experiences and drug use (e.g. tobacco, drugs of abuse), in which there were no significant differences between both sexes (14, 15).
3.3. Adverse life events
Those who experienced a higher number of adverse life events were more likely to use drugs, starting at an early age, and to suffer from schizophrenia in adulthood (13). The article published by Low et al. (16) intended to make a connection between daily stress, mental health and drug use in adolescence. This article concludes that, according to the statistics, breakup stress was significantly associated with the relationship between mental health and drug use (except for illicit drugs use). Family dysfunctionality was significantly associated as well with depression symptoms, marijuana use, and cigarette use.
3.4. Socioeconomic situation
The article written by Marilis Vaht et al. (13) indicated that the association between cannabis use and the subsequent development of schizophrenia was only shown in the cohort of younger subjects. Consequently, the authors concluded that it was necessary to take into account the socioeconomic situation of the country during the period in which the study was conducted to interpret this association. Studies conducted in most of Latin-American countries, likewise to those conducted in countries with higher income, showed divergent results in relation to the association between the individual socioeconomic status and the presence of mental disorders. Nevertheless, a more frequent use of illicit drugs, such as marijuana and cocaine, has been observed among people with a lower socioeconomic status (17).
- Discussion
4.1. Source reliability
We based our research on cohort studies and their reviews. Several of the results shown in the sources could be limited because their data only regarded cannabis use before conscription (10, 18) or because their data was inconsistent and inadequately standardized (19). Therefore, these sources could reflect a type of information bias.
On the other hand, the diagnosis was based on standardized and official surveys, whilst the surveys about cannabis use were not. Another source of information bias could be the fact that the self-reported surveys were not anonymous, although the error could be higher by using anonymous questionnaires (10, 20). In addition, this could lead to an anamnestic bias, underestimating drug use by non-psychotic patients.
The study Trends in cannabis use prior to first presentation with schizophrenia, in South-East London between 1965 and 1999 (21) was conducted by studying patients with non-psychotic psychiatric disorders. This explains why the sample regarding cannabis use could not have been representative of the general population and, consequently, why it could have underestimated certain values. The reviews were based on large cohort studies that included well-designed models (22, 24)
4.2. Cause-effect relationship
The hypothesis considering cannabis use as causal factor of schizophrenia is being questioned today. Many researchers consider that this statistical association could be caused by confounding factors. On the contrary, a great quantity of scientific evidence has supported this relationship. For instance, important cohort studies suggest that cannabis could have a dual effect, having a direct causality relationship with shared vulnerabilities.
Regarding the confounding factors, we based our study on research focused on socioeconomic and psychological aspects of the population and other variables dependent on each person (e.g. sex, ethnic group, age). In both types of research, even though variables such as being a man or being young suggested a higher drug use, the most confounding variable was the diagnosis of schizophrenia before the scientific conscription. However, once this effect was mitigated, the relationship between cannabis and schizophrenia was still significant.
Cannabis and dopamine biological mechanisms (22), as well as the discoveries that relate the NRG1 polymorphism with drug abuse (13), indicate a plausible biological mechanism that reinforces the hypothesis. Furthermore, the dosis-effect relationship between cannabis and its psychotic symptoms and a temporal sequence between previous cannabis use and development of schizophrenia have been observed in several studies (23). Probably, precocious schizophrenia entails a higher cannabis use (25), but studies have concluded exactly the contrary (19).
According to the study What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? (23), regular cannabis users double their risks of experiencing psychotic symptoms and disorders, especially if they have a personal or familiar history of psychotic disorders, and if they initiate cannabis use in their adolescence. However, it is important to highlight that, despite the rise of cannabis use over the last decades, the number of patients with schizophrenia remains stable (19).
- Conclusion
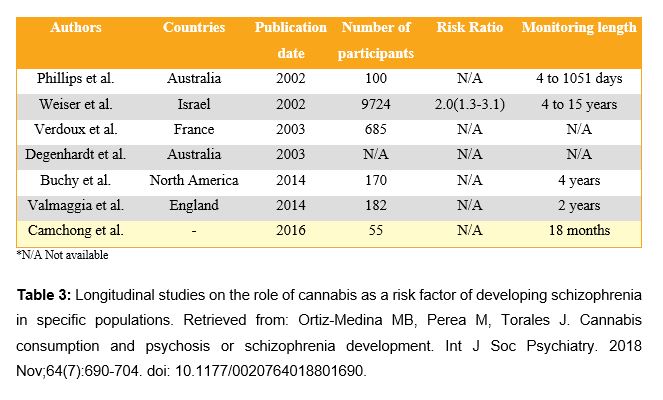
Nowadays, the question about the role of cannabis in schizophrenia disorder has been widely explored, mainly by cohort studies or longitudinal studies, as shown in Table 3 (26). Regarding the discussion on cannabis legalization, it is relevant to point out its role in schizophrenia pathology. According to Grant S. (22), it seems that there is enough evidence to state that cannabis takes part in certain causal mechanisms of schizophrenia. However, it was estimated that, in order to prevent a single case of psychosis induced by cannabis, it would be necessary that more than 1,300 men aged 20-24 were prevented from becoming heavy cannabis users. In conclusion, it is possible to declare that there is a relationship between cannabis use and the development or worsening of schizophrenic episodes.
Conflicts of interest statement
The authors declare that there are no conflicts of interest in this article.
References
- Carpenter Jr WT et al. Espectro de la esquizofrenia y otros trastornos psicóticos. In: Kupfer DJ, Regier DA, Narrow WE, Schultz SK, Kuhl EA, Blazer DG. Manual diagnóstico y estadístico de los trastornos mentales: DSM-5. 5th ed. Madrid: Panamericana; 2016. p. 87-122.
- Bobes J, Bascarán MT, González MP, Sáiz PA. Epidemiología del uso/abuso de cannabis. Adicciones 2000;12:31-40. doi: 10.20882/adicciones.671.
- Álvarez E, Brime B, Llorens N, Molina M, Sánchez E, Sendino R. Estadísticas 2018 Alcohol, tabaco y drogas ilegales en España Encuesta sobre uso de drogas en enseñanzas secundarias en España (ESTUDES), 1994-2016. [online]. Madrid: Ministerio de sanidad, consumo y bienestar; 2018. [last access: 20/03/2019]. Available at: http://www.pnsd.mscbs.gob.es/profesionales/sistemasInformacion/sistemaInformacion/pdf/ESTUDES_2018_Estadisticas.pdf
- Fischer BA, Buchanan RW. Schizophrenia in adults: Epidemiology and pathogenesis [online]. Feb, 2019, Mar 13, 2019. Uptodate. https://www.uptodate.com/
- Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol. Psychiatry. 2017;81(1): 31–42. doi:10.1016/j.biopsych.2016.03.2104.
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4-6):365-84. doi: 10.1007/s10571-006-9062-8.
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107-20. doi: 10.1038/nrn3155.
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188(6):510-8. doi: 10.1192/bjp.188.6.510.
- Sam Wang G. Cannabis (marijuana): Acute intoxication [online]. Feb, 2019 Feb 26, 2019. Uptodate. http://www.uptodate.com/
- Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and Schizophrenia: A Longitudinal Study of Swedish Conscripts. Lancet. 1987;330(8574):1483-486. doi: 10.1016/B978-0-12-804791-0.00011-2.
- Lovell ME, Bruno R, Johnston J. Cognitive, physical, and mental health outcomes between long-term cannabis and tobacco users. Addict Behav. 2018;79:178-188. doi: 10.1016/j.addbeh.2017.12.009.
- Khokhar JY, Dwiel LL, Henricks AM, Doucette WT, Green AI. The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophr Res. 2018;194:78-85. doi: 10.1016/j.schres.2017.04.016.
- Vaht M, Laas K, Kiive E, Parik J, Veidebaum T, Harro J. A functional neuregulin-1 gene variant and stressful life events: Effect on drug use in a longitudinal population-representative cohort study. Psychopharmacol. 2017;31(1): 54–61. doi: 10.1177/0269881116655979.
- Booker CL, Unger JB, Azen SP, Baezconde-Garbanati L, Lickel B, Johnson CA. A longitudinal analysis of stressful life events, smoking behaviors, and gender differences in a multicultural sample of adolescents. Subst Use Misuse. 2008;(43):1509-1531. doi: 10.1080/10826080802238009.
- Nolen-Hoeksema. Emotion regulation and psychopathology: the role of gender. Annu. Rev. Clin. Psychol. 2012; 8:161–87. doi: 10.1146/annurev-clinpsy-032511-143109.
- Low NCP, Dugar E, O’Loughlin E et al. Common stressful life events and difficulties are associated with mental health symptoms and substance use in young adolescents. BMC Psychiatry. 2012;12(116): 1-10. doi: 10.1186/1471-244X-12-116.
- Ortiz-Hernandez L, Lopez-Moreno S, Borges G. Desigualdad socioeconómica y salud mental: revisión de la literatura latinoamericana. Cad. Saúde Pública. 2007;23(6):1255-1272. doi: 10.1590/S0102-311X2007000600002.
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325(23):1195-1212. doi: 10.1136/bmj.325.7374.1199.
- Hickman M, Vickerman P, Macleod J, Kirkbride J, Jones PB. Cannabis and schizophrenia: model projections of the impact of the rise in cannabis use on historical and future trends in schizophrenia in England and Wales. Addiction. 2007;102(4):597-606. doi: 10.1111/j.1360-0443.2006.01710.x.
- Benson G, Holmberg MB. Validity of questionnaires in population studies on drug. Acta Psychiatr Scand. 1985.71(1):9-18. doi: 10.1111/j.1600-0447.1985.tb05045.x.
- Boydell J, van Os J, Caspi A et al. Trends in cannabis use prior to first presentation with schizophrenia, in South-East London between 1965 and 1999. Psychol Med. 2006 Oct;36(10):1441-6. doi: 10.1017/S0033291706008440.
- Grant S. Cannabis, stimulants and psychosis. Commentary on Gururajan et al. (2012): drugs of abuse and increased risk of psychosis development. ANZJP Correspondence. 2012;46(12):1196-7. doi: 10.1177/0004867412459812.
- Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110(1):19-35. doi: 10.1111/add.12703.
- Gururajan A, Manning EE, Klug M, van den Buuse M. Drugs of abuse and increased risk of psychosis development. Aust N Z J Psychiatry. 2012;46(12):1120-35. doi: 10.1177/0004867412455232.
- Macleod J, Oakes R, Copello A et al. Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet. 2004;363(9421):1579–88. doi: 10.1016/S0140-6736(04)16200-4.
- Ortiz-Medina MB, Perea M, Torales J. Cannabis consumption and psychosis or schizophrenia development. Int J Soc Psychiatry. 2018 Nov;64(7):690-704. doi: 10.1177/0020764018801690.