Patricia López-Gómez 1; Manuel González-Alcaide 2
1 Research Group of Biomaterials, Biomechanics and Tissue engineering (BBT), Polytechnic University of Catalonia (UPC)
2 Faculty of Medicine, University of Granada (UGR)
Translated by:
Celia Amat-Galdeano 3; Paula Gómez-Martín 3; Alicia Martínez-Martínez 3; Marta María Pérez-Puerta 3; Aïcha Rabbaj 3; Inés San Juan-Sánchez 3
3 Faculty of Translation and Interpreting, University of Granada (UGR)
The spine is a key part of the structure of our organism. Due to its length, location and composition, it is exposed to numerous factors that may reduce the quality of life of patients. Tissue engineering is an emerging area of biomedical research that combines the principles of engineering, biology and medicine in order to create morphofunctional substitutes for tissues and organs. This discipline has experienced exponential technological advances over the last two decades thanks to the development of three-dimensional (3D) printing and bioprinting. Given this unprecedented growth, the authors have decided to elaborate a bibliographic revision of the advances in the production of biomodels and guides for surgical planning, as well as in the production of intervertebral cartilage substitutes by tissue engineering. This revision will focus on 3D bioprinting techniques, which are a very promising tool in the treatment of a large number of spine diseases.
Keywords: 3D printing, bioprinting, intervertebral cartilage, spine, tissue engineering
Introduction
The spine is a key part of the structure of our organism. It is exposed to numerous stress factors, such as mechanical traction, trauma, and other diseases, due to its length, location, and composition, which may reduce the quality of life of the patient (1).
The degenerative disease of the spine is a major cause of global disability, with around 266 million people suffering from it each year (2). It includes diverse pathologies like spondylolisthesis, intervertebral disc degeneration or lumbar spinal stenosis (3), which cause a wide range of diseases such as lower limb pain, weakness, and serious low back pain. Spine pathologies significantly reduce the quality of life of patients (2, 4).
The structural and functional complexity of the spine, together with the vascularity and poor regenerative capacity of the cartilaginous tissue of the intervertebral disc have caused the necessity of seeking new therapies in tissue engineering, different from traditional methods which are currently ineffective. Among these traditional methods are, pharmacological techniques (analgesics, steroids, non-steroidal anti-inflammatories…) or procedures (placing steel rods to try to correct curvatures of the spine, decompression and fusion techniques…) (5, 6), which are currently ineffective (7).
Tissue engineering is an emerging area of biomedical research that combines the basic principles of engineering, biology, and medicine in order to create functional substitutes for therapeutic use (8). This discipline has experienced exponential technological advances in recent years as evidenced by the development of three-dimensional (3D) printing and bioprinting of tissues and organs (9-11). Nowadays, many of these materials and advanced therapy products have already been successfully put into clinical practice (12). It is possible to produce artificial tissues that resemble the structure and function of native tissues, including cartilaginous tissue (13), by using and combining cells, biomaterials, and bioactive factors, and through the use of an optimal biofabrication technique.
The aim of this paper is to elaborate a bibliographical revision of the current state of 3D printing techniques, discuss their possible applications in tissue engineering and, more particularly, in the repair of spine structures severely damaged by stress or by any secondary defect due to a disease. The biological basis of the tissues under study and current treatments (in use) will be presented below. Advances in models and guides for surgical planning and the production of intervertebral cartilage substitutes by tissue engineering and bioprinting will also be described. Lastly, future research strategies and their possible clinical translation will be discussed.
Biological basis of bone and cartilaginous tissues of the spine
The embryonic origin of the entire vertebral structure is mesodermal and endodermal: a major part of the spine is derived from the mesoderm, together with the vertebrae, the end plates of chondral tissue and the annulus. In contrast, the nucleus pulposus is originated in the endoderm.
The development of the spine starts during the gastrulation process, when the mesoderm surrounding the notochord is divided into three areas: paraxial, lateral, and intermediate. The paraxial mesoderm is differentiated into 42 pairs of somites, each divided into a dermomyotome and a sclerotome. The sclerotome becomes the skeleton of the spine (14).
Histology of the vertebra and the intervertebral disc reveals highly specialized and organized tissues that are well integrated with each other. However, it can suffer from mechanical deterioration and morphophysiological changes that contribute to diseases when aging and injuring (3,15).
The intervertebral disc presents a chondral structure, which resembles other cartilaginous tissues in terms of biochemistry, although it significantly differs from these tissues in morphological terms (3). It is the first connective tissue that manifests signs of deterioration and aging (16). Therefore, the safest and most effective way to treat an injury requires a deep understanding of the structure, composition, and cellular organization.
Advanced therapies for the treatment of spine injuries
Loss of structure, deterioration of the cartilage matrix and/or lack of integrity of the vertebra itself may occur during a spine injury (3,13). Locomotion problems derived from the loss of spinal cord alignment, flexibility or neural anatomy may lead to diseases that reduce the quality of life of the patient (16), such as herniation (disc protrusion or disc extrusion), stenosis, osteophytes, spondylolisthesis, and spondylosis. These diseases have become highly relevant in biomedical research, contributing to the development of many palliative and reparative techniques in the treatment of spine injuries, especially cellular therapy and tissue engineering (3,17).
Cellular therapy
Cellular therapy refers to the treatments that use cells as the main active component to treat diseases or pathologies (18). These techniques have a great potential in the treatment of spine injuries and have become an alternative to traditional techniques or prosthetic replacements, which translates into an improvement in the quality of life of patients (13).
Tissue engineering
Due to the limitations attached to traditional techniques, a variety of combinatorial strategies between cells, biomaterials and signalling molecules (8) have been suggested. Some of these strategies include the use of cell-loaded hydrogels (12), scaffold-based implants that promote cell recruitment with signalling molecules (without external cellular input), techniques based on the production of biomaterial-free substitutes (17). These strategies may also include additional gene therapy methods to express specific growth factors (13).
3D printing applied in tissue engineering is one of the technologies proposed to improve and optimize the treatments for this type of injury. It could be an alternative to traditional methods, which are currently inefficient (19).
Basic concepts of 3D printing
3D printing is a prototyping and additive production technique used in the construction of complex architectural models, that is, a high-precision mechanism that is accomplished through a process of successive addition of layers of the material concerned. This technique facilitates the production and replication of complex structures with high precision and in a controlled way, considering factors such as external shape, internal geometry, porosity, and interconnectivity. Simultaneously, this process enables high reproducibility and repeatability of the results obtained (20).
This idea was first introduced in the 1970s by Pierre A. L. Ciraud, who described a production method in which the solidification of layers (21) was achieved by sputtering material and a high-energy beam.
A decade later, StereoLithography Apparatus (SLA) became the first additive production technique applied in medicine for the surgical model of alloplastic implants (22, 23). One of the first works in tissue engineering using this technology for peripheral nerves regeneration (24) was conducted by Widmer and his group in the 1990s. In the same decade, Emanuel Sachs and his group also patented “three-dimensional printing techniques” for medical application (25).
In the last ten years, multiple printing techniques have been optimized. Multi-head printers have been developed in order to print different materials simultaneously. Furthermore, the use of medical imaging techniques and computer-aided design software has been introduced (21, 26, 27).
3D printing techniques:
Additive manufacturing technologies can be classified into four different techniques.
- Vat polymerization (VAT) based printing technique
A molten polymer previously located in a vat is polymerized by a light source (figure 1.1). This process may be performed with techniques such as SLA techniques, Digital Light Processing (DLP) and Continuous Digital Light Processing (CDLP) can be found.
- Powder-based 3D printing technique
A laser goes through the area where the material has been deposited, fusing the dust particles into successive layers (figure 1.2). This process may be performed with the techniques of Selective Laser Sintering (SLS), Direct Metal Laser Sintering (DMLS), Selective Laser Melting (SLM) and Electron Beam Melting (EBM).
- Droplet-based printing technique (inkjet)
A precise stream of liquid material is directed towards a substrate in order to build a model by layering (figure 1.3). This process may be performed with the techniques of Multi Jet Modeling (MJM), the Wax Deposition Modeling (WDM), the Laser-Induced Forward Transfer (LIFT) and the Binder Jetting (BJ).
- Extrusion-based 3D printing technique
A molten polymer is deposited through the extruder of the 3D printer, which layers the material over the substrate (figure 1.4). This method uses the techniques of Fused Deposition Modeling (FDM) and Direct Ink Write (DIW) (21, 22, 28, 29).
Figure 1. Graphical representation of the different techniques used in 3D printing.
- Vat polymerization-based printing. 2. Powder-based 3DP (3D printing). 3. Droplet-based printing. 4. Extrusion-based printing.

Technical fundamentals of 3D printing regarding the repair of spine diseases
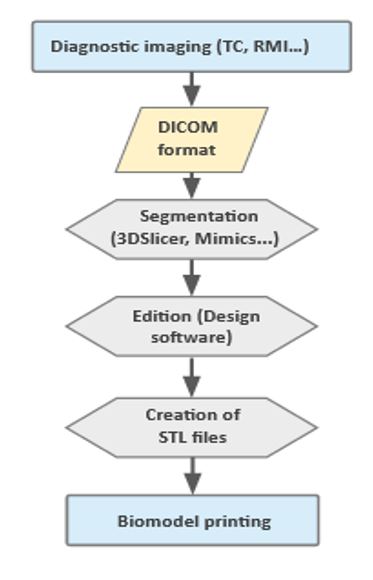
Creation of anatomical models based on DICOM images for chirurgical planification
The first step in order to obtain information regarding the current state of the disease and create an action protocol is an imaging diagnostic. This may be performed with the techniques of Computed Tomography (CT) or Magnetic Resonance Imaging (MRI). The information of the images will be collected afterwards in DICOM (Digital Imaging and Communication on Medicine) format. This technique will be used to obtain a computerized design with a Computer-Aided Design (CAD) software and a particular segmentation programme. Lastly, a layering file will be generated in STL (STereoLithography) format in order to be sent to the 3D printer (7) (figure 2). The resulting printed biomodels provide very accurate information about the specific status of the patients’ disease, which can represent an extraordinary support in surgery planning, as it can help lessen the time and possible risks related to the procedure (30).
Figure 2. Flowchart of the required steps in order to create an anatomical model based on medical images with 3D printing techniques.
Printing of biomaterials in tissue engineering
Biomaterials are essential in tissue engineering. They provide structural support for cells to adhere, migrate, proliferate, produce their own extracellular matrix (ECM) and distinguish themselves in a specific phenotype (22). This phenotype must be biocompatible, bioactive, and porous, and have a proper mechanical strength. Moreover, the biodegradability of the biomaterial is key to the progressive replacement of artificial tissue for neoformed tissue (31,32).
Conventional techniques such as electrospinning (33), lixiviation, lyophilization or moulding (27) have been commonly used. Nevertheless, these techniques lack the precision required in order to control the porosity, internal geometry and spatial arrangement of the components (22).
Additive manufacturing has become more relevant as an alternative way of fabricating this structural support. This allows the control of the size, shape and architecture of the scaffold, as well as a high level of precision, which is necessary in order to tune the porosity and degradability, and the tridimensional distribution of the elements (27,33). Other advantages are its customized individual design, its high reproducibility and its efficiency. The most employed techniques are FDM (21), SLS/DMLS, BJ and VAT (22).
Usage of 3D printing technologies regarding spine diseases.
3D printing has been used in various spine diseases. The most remarkable uses are the fabrication of individualized implants with high rates of osseointegration and anatomical precision, the lateral lumbar interbody fusion in a patient with intractable radiculopathy due to disc compression (34), the removal of a T9 destructive bone tumor in a patient with progressive kyphoscoliosis (35), and the treatment of symptomatic cysts in the sacral canal by the use of artificial printed dura mater among with radiculopathy (36).
3D bioprinting
The ideal approach towards additive fabrication for scaffolds includes cell incorporation, which may be performed either by sowing them in the biomaterial once it has been printed or by incorporating them directly as printing material. Any technique including cells in ink formulation different to the previously mentioned 3D printing techniques is called bioprinting (22).
Bioprinting enables the creation of tridimensional compound structures with biomaterials, which act as a support, and cellular components (29, 37). It is also possible to add other elements as pharmacs, components of the ECM, and growth and other biological factors (38) in order to create tissues that resemble native tissue or biomimetics (29). The three most used technologies are bioink injection, extrusion and light-assisted bioprinting (38).
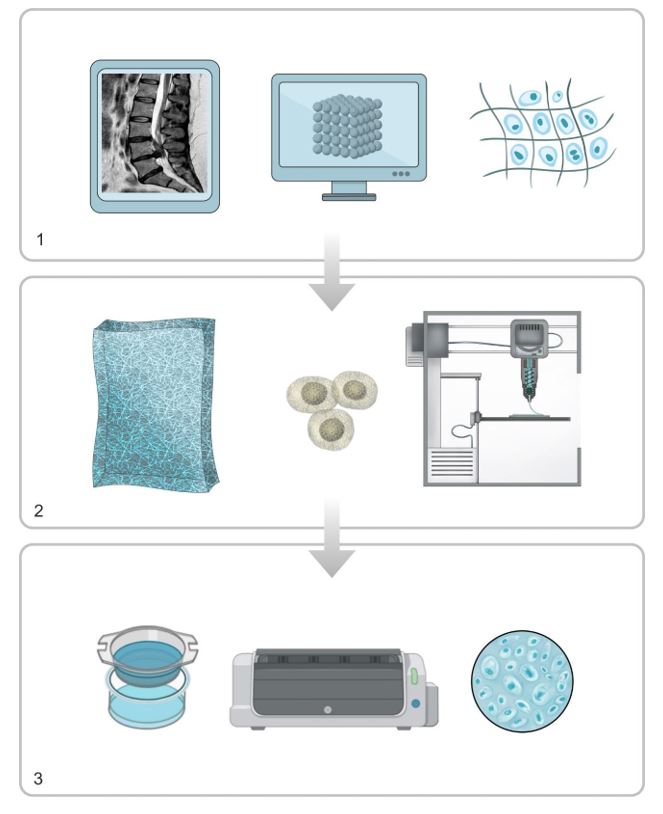
The process of 3D bioprinting is divided into 3 different phases.
Pre-processing. The 3D object is designed with a CAD software and data is characterized in order to optimize geometry. Pre-processing implies the digital design obtained with medical images (CT or RMI) and the selection of components (figure 3.1).
Processing. The generation of layers and printing process of the model is performed with the selected technique. Designed images are sent to the system. Biomaterials and bioinks are prepared (figure 3.2).
Post-processing. Adaptation of the printed tissue for its subsequent use. This process can take days or even weeks, since printed constructions are transferred to a bioreactor for tissue maturation (figure 3.3) (21, 27, 29, 39).
Figure 3. Graphical representation of the general bioprinting process.
- Pre-processing. 2. Processing. 3. Post-processing.
Creation of cartilaginous substitutes by 3D bioprinting techniques
The challenges associated with the architecture and complexity of chondral tissue require making faultless decisions on the components and techniques related to biofabrication (28). The interactions between the cells and the scaffold are determining for the quality of the final result of the printed model. Therefore, choosing the best option for every single element is crucial (29).
Biomaterials
The biomaterials used in cartilage bioprinting can be of natural or synthetic origin, or a mixture of both, resulting in improved biomechanical and biological properties (28, 29, 40).
The most common natural polymers used are: collagen, the most abundant component in the extracellular matrix of mammals; agarose, a thermosensitive polymeric hydrogel; alginate, derived from the wall of brown algae; chitosan, polysaccharide that can form a gel matrix; hyaluronic acid (HA), anionic polysaccharide that promotes chondral tissue regeneration; and gelatin (28, 29, 41). Their main benefit is their biocompatibility and low cytotoxicity. However, they possess a low manipulation capacity and printing resolution due to their viscosity (41).
To overcome these limitations, synthetic polymers are used in the development of hydrogels since, unlike natural polymers, their properties can be controlled. This gives them some advantages in terms of mechanical strength, stability, manipulation and even biodegradability (40). Some of the synthetic polymers most commonly used are: poly(vinyl alcohol) (PVA), poly(ethylene) glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), and poly-caprolactone (PCL) (29).
Taking into account the properties of each type, a mixed hydrogel created with a natural and a synthetic component solves these limitations. As a result, improved biomechanical and biological properties are obtained, which exceeds the results achieved separately (40). For this reason, composite scaffolds are usually used.
The most common combinations are:
-The combination of alginate with nanocellulose (19, 29), as it has shown really favorable properties related to the construct stabilization and viscosity decrease (41).
-The combination of chitosan and poloxamers compounds favors the growth of chondrocytes, and the combination of those compounds with acrylate produces a nanostructured hydrogel.
-The combination of chitosan (80%), PCL (15%) and hydroxyapatite (5%) produce bioscaffolds that allow the emulation of characteristics resembling human cartilage and are capable of being subjected to a histological processing (42).
-The combination of side groups with either gelatin hydrogels or HA in order to facilitate crosslinking between polymers, which results in a very stable matrix (43).
-The combination of PEG hydrogels with either peptides or adhesive proteins in order to confer them physiological cell-material interaction abilities, etc. (28, 29).
Cellular sources
Although the use of differentiated chondrocytes in bioprinting has been widely described (19, 29, 44, 45), it is difficult to obtain them directly due to their low renewal rate, the limitation of extraction sources and the inherent complexity of their collection. As a result, new alternative cellular sources have been tested, such as stem cells (41). These cells form a group of undifferentiated cells with an unlimited capacity of division and a high regeneration potential.
Depending on their differentiation potential, stem cells are classified as totipotent, pluripotent, multipotent, or unipotent. According to their origin, they are classified as embryonic stem cells, fetal stem cells, or “adult” stem cells (somatic) (8, 17), the latter being the most commonly used. The use of Mesenchymal Stem Cells (MSCs) implies efficacy and safety for the patient (19, 46), posing a lower risk in the formation of teratomas or unwanted differentiation compared to the action of embryonic stem cells (41). They can be obtained from a large number of tissues. Those derived from bone marrow and adipose tissue (8) are the most widely used in chondral tissue treatments (28, 46), as they show an excellent chondrogenic potential (47). Adipose tissue cells are really promising for the treatment of musculoskeletal disorders, such as the adipose tissue is a huge source of stem cells for several reasons: they are very abundant in the human organism, they can be obtained easily from the patient through minimally invasive procedures, and they have great efficiency at cellular level (48). In addition, several studies have demonstrated that the treatment with co-cultures produces better results than those performed with monocultures, being the mixture of MSCs and chondrocytes in a 4:1 ratio the most efficient one (41).
Limitations of 3D printing technologies
These limitations refer to the lack of resolution of the 3D image and the presence of devices during the generation of the 3D model. In some cases, these devices affected the architecture of manufactured prostheses, making them totally unusable. It is also important to mention the high cost of all the equipment, the printing speed and the lack of qualified professionals who know how to operate the printers during emergencies (49).
Future prospects of 3D printing technologies for the treatment of spinal pathologies
3D printing technology was described decades ago, but bioprinting is much more recent. Although it evolves quite fast, showing ability and flexibility to generate living tissues with minimal or no side effects, in vivo studies are still at an early stage. There is a gap between the biological and mechanical properties that must be bridged (29), as it is essential to stabilize the implant and integrate it with the surrounding native tissue (45).
However, the advances and results obtained allow optimism about 3D bioprinting of complex systems and the solution of shortage of tissue and organ donors (29, 50).
Clinical translation
3D printing technology is already part of a number of clinical routines, such as the generation of models based on reference images taken via CT or MRI, and the fabrication of prototypes for educational purposes to assist surgeons in the planning of complex surgeries (50).
For the clinical translation of bioprinting, safety standards and requirements must be met: sterility, endotoxin content and reproducibility. Furthermore, due to the presence of cells, this translation must be subject to the corresponding law, incorporating good manufacturing practices, ensuring minimal manipulation and closed-system processing.
However, there are no current updated regulations that evaluate quality, safety and efficiency of 3D bioprinting in patients. Up to now, only recommendations for the preclinical and clinical studies have been provided by the International Cartilage Regeneration & Joint Preservation Society (29, 50).
Conclusions
3D printing technologies could offer great advantages for the patient, such as the development of a totally personalized treatment capable of accurately solving highly complex injuries. This would make possible facing future surgical operations with greater safety and prior knowledge.
As for 3D bioprinting, there are obstacles that need to be overcome before it can be used in clinically relevant environments. It is necessary to evaluate all the possible combinations between biomaterials, cell sources and molecules in order to establish a perfect construction that makes possible an optimal cartilage substitute, both in structure and function. For this purpose, it is essential to carefully select materials with adequate cell compatibility and mechanical properties.
Regulation is also needed at all levels, from the legislative level, considering cell-based biomanufacturing techniques, to the use of materials for guides and implants generated by 3D printing in order to ensure a correct and safe manufacture and application for the patient.
Despite these limitations, it is enlightening that the future of spine injuries management lies in the development of interdisciplinary techniques, which bring together the capabilities of regenerative medicine and bioengineering. The next generation of biologically compatible implants, capable of treating spinal damage, will become a reality thanks to the implementation of 3D printing in hospitals as an alternative to current techniques.
Statements
Acknowledgements
The authors of this paper would like to thank the involvement of the coordinating and teaching staff of the “Producción y traducción de artículos científicos biomédicos (III ed.)” and the “Traducción inversa de artículos científicos biomédicos (español-inglés)” courses, as well as the English translation team.
Conflicts of interests
The authors declare no conflict of interest.
References
- Artificial discs for lumbar and cervical degenerative disc disease -update: an evidence-based analysis. Ont Health Technol Assess Ser. 2006;6(10):1–98.
- Ravindra VM, Senglaub SS, Rattani A, Dewan MC, Härtl R, Bisson E, et al. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Glob spine J. 2018;8(8):784–94.
- Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54–67.
- Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(6):968–74.
- Kalichman L, Hunter DJ. Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2008;17(3):327–35.
- Cunin V. Early-onset scoliosis: current treatment. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S109-18.
- Cho W, Job AV, Chen J, Baek JH. A Review of Current Clinical Applications of Three-Dimensional Printing in Spine Surgery. Asian Spine J. 2018;12(1):171–7.
- Hunziker EB, Lippuner K, Keel MJB, Shintani N. An educational review of cartilage repair: precepts & practice–myths & misconceptions–progress & prospects. Osteoarthr Cartil. 2015;23(3):334–50.
- Lippincott Williams & Wilkins. Histology for pathologists. MILLS S, editor. 2019.
- Sheha ED, Gandhi SD, Colman MW. 3D printing in spine surgery. Ann Transl Med. 2019;7(Suppl 5):S164.
- Burnard JL, Parr WCH, Choy WJ, Walsh WR, Mobbs RJ. 3D-printed spine surgery implants: a systematic review of the efficacy and clinical safety profile of patient-specific and off-the-shelf devices. Eur Spine J [Internet]. 2020;29(6):1248–60. Disponible en: https://doi.org/10.1007/s00586-019-06236-2
- Bonhome-Espinosa AB, Campos F, Durand-Herrera D, Sánchez-López JD, Schaub S, Durán JDG, et al. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J Mech Behav Biomed Mater. 2020;104:103619.
- Álvarez E, Ripoll PL, Restrepo A, Forriol F. Revisión de la reparación del cartílago. Posibilidades y resultados. Trauma (Spain). 2010.
- Kalamchi L, Valle C. Embryology, Vertebral Column Development. In Treasure Island (FL); 2021.
- Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88 Suppl 2:10–4.
- Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–30.
- Caldwell KL, Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthr Cartil. 2015;23(3):351–62.
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10.
- Daly AC, Freeman FE, Gonzalez-Fernandez T, Critchley SE, Nulty J, Kelly DJ. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv Healthc Mater. 2017;6(22).
- Cui H, Nowicki M, Fisher JP, Zhang LG. 3D Bioprinting for Organ Regeneration. Adv Healthc Mater. 2017;6(1).
- Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D Printing in Pharmaceutical and Medical Applications – Recent Achievements and Challenges. Pharm Res. 2018;35(9):176.
- Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017.
- Anderl H, Zur Nedden D, Mühlbauer W, Twerdy K, Zanon E, Wicke K, et al. CT-guided stereolithography as a new tool in craniofacial surgery. Br J Plast Surg. 1994;47(1):60–4.
- Widmer MS, Gupta PK, Lu L, Meszlenyi RK, Evans GR, Brandt K, et al. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials. 1998;19(21):1945–55.
- Haggerty J, Cima M, Williams P, inventores; Sachs E, titular. (1993). Técnicas de Impresión Tridimensional. Patente Estadounidense. 5,204,055 04/28/1993.
- Richter DL, Schenck RCJ, Wascher DC, Treme G. Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health. 2016;8(2):153–60.
- Sabino MA, Loaiza M, Dernowsek J, Rezende R, Da Silva JVL. Técnicas para la fabricación de andamios poliméricos con aplicaciones en ingenierÍa de tejidos. Rev Latinoam Metal y Mater. 2017.
- Vijayavenkataraman S, Yan W-C, Lu WF, Wang C-H, Fuh JYH. 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev. 2018;132:296–332.
- Huang Y, Zhang XF, Gao G, Yonezawa T, Cui X. 3D bioprinting and the current applications in tissue engineering. Biotechnology Journal. 2017.
- D’Urso PS, Williamson OD, Thompson RG. Biomodeling as an aid to spinal instrumentation. Spine (Phila Pa 1976). 2005;30(24):2841–5.
- Moroni L, Boland T, Burdick JA, De Maria C, Derby B, Forgacs G, et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018;36(4):384–402.
- Elkasabgy NA, Mahmoud AA. Fabrication Strategies of Scaffolds for Delivering Active Ingredients for Tissue Engineering. AAPS PharmSciTech. 2019;20(7):256.
- Sun J, Vijayavenkataraman S, Liu H. An Overview of Scaffold Design and Fabrication Technology for Engineered Knee Meniscus. Mater (Basel, Switzerland). 2017;10(1).
- Siu TL, Rogers JM, Lin K, Thompson R, Owbridge M. Custom-Made Titanium 3-Dimensional Printed Interbody Cages for Treatment of Osteoporotic Fracture-Related Spinal Deformity. World Neurosurg. 2018;111:1–5.
- Choy WJ, Mobbs RJ, Wilcox B, Phan S, Phan K, Sutterlin CE 3rd. Reconstruction of Thoracic Spine Using a Personalized 3D-Printed Vertebral Body in Adolescent with T9 Primary Bone Tumor. World Neurosurg. 2017;105:1032.e13-1032.e17.
- Liu B, Wang Z, Lin G, Zhang J. Radiculoplasty with reconstruction using 3D-printed artificial dura mater for the treatment of symptomatic sacral canal cysts: Two case reports. Medicine (Baltimore). 2018;97(49):e13289.
- Fricain JC, De Olivera H, Devillard R, Kalisky J, Remy M, Kériquel V, et al. Impression 3D en médecine régénératrice et ingénierie tissulaire. Médecine/Sciences. 2017; 33 (1) 52-59.
- Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Current Opinion in Biotechnology. 2016.
- O’Brien CM, Holmes B, Faucett S, Zhang LG. Three-Dimensional Printing of Nanomaterial Scaffolds for Complex Tissue Regeneration. Tissue Eng Part B Rev [Internet]. 2015;21(1):103–14.
- Roseti L, Cavallo C, Desando G, Parisi V, Petretta M, Bartolotti I, et al. Three-Dimensional Bioprinting of Cartilage by the Use of Stem Cells: A Strategy to Improve Regeneration. Mater (Basel, Switzerland). 2018;11(9).
- Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, et al. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS One. 2017;12(12):e0189428.
- Láinez Ramos AJ, Rivera Izquierdo M. Synthesis and characterization of a cartilage model using hydroxyapatite, chitosan and polycaprolactone. Actual Medica. 2017;102(800):7–12.
- Duchi S, Onofrillo C, O’Connell CD, Blanchard R, Augustine C, Quigley AF, et al. Handheld Co-Axial Bioprinting: Application to in situ surgical cartilage repair. Sci Rep. 2017;7(1):5837.
- Shim JH, Lee JS, Kim JY, Cho DW. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromechanics Microengineering. 2012;
- Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11–12):1304–12.
- Lopa S, Mondadori C, Mainardi VL, Talò G, Costantini M, Candrian C, et al. Translational Application of Microfluidics and Bioprinting for Stem Cell-Based Cartilage Repair. Stem Cells Int. 2018;2018:6594841.
- Żylińska B, Silmanowicz P, Sobczyńska-Rak A, Jarosz Ł, Szponder T. Treatment of Articular Cartilage Defects: Focus on Tissue Engineering. In Vivo. 2018;32(6):1289–300.
- Rivera-Izquierdo M, Cabeza L, Láinez-Ramos-Bossini A, Quesada R, Perazzoli G, Alvarez P, et al. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin Biol Ther. 2019;19(3):233–48.
- Martelli N, Serrano C, van den Brink H, Pineau J, Prognon P, Borget I, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery. 2016;159(6):1485–500.
- Mouser VHM, Levato R, Bonassar LJ, D’Lima DD, Grande DA, Klein TJ, et al. Three-Dimensional Bioprinting and Its Potential in the Field of Articular Cartilage Regeneration. Cartilage. 2017;8(4):327–40.
AMU 2021. Volumen 3, Número 1
Fecha de envío:
14/03/2021
Fecha de aceptación:
04/04/2021
Fecha de publicación:
31/05/2021